8.2: Halogenation of Alkenes - Addition of X₂
- Page ID
- 67137
Objectives
After completing this section, you should be able to
- write the equation for the reaction of chlorine or bromine with a given alkene.
- identify the conditions under which an addition reaction occurs between an alkene and chlorine or bromine.
- draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine.
- write the mechanism for the addition reaction that occurs between an alkene and chlorine or bromine, and account for the stereochemistry of the product.
Make certain that you can define, and use in context, the key terms below.
- anti stereochemistry
- bromonium ion
In the laboratory you will test a number of compounds for the presence of a carbon-carbon double bond. A common test is the decolourization of a reddish-brown bromine solution by an alkene.
The two-step mechanism shown in the LibreText pages gives you an idea of how the reaction between an alkene and a halogen occurs. Note the formation of the bridged bromonium ion intermediate and the anti stereochemistry of the final product because the two bromine atoms come from opposite faces of the double bond.
Additional evidence in support of the bromonium ion mechanism comes from the results obtained when an alkene (such as cyclopentene) reacts with bromine in the presence of sodium chloride (see Figure 8.2: Reaction of an alkene with bromine in the presence of sodium chloride, below).
Figure 8.2: Reaction of an alkene with bromine in the presence of sodium chloride
Once formed, the bromonium ion is susceptible to attack by two nucleophiles—chloride ion and bromide ion—and, in fact, a mixture of two products (both produced by anti attack) is formed.
Halogens can act as electrophiles to attack a double bond in alkene. Double bond represents a region of electron density and therefore functions as a nucleophile. How is it possible for a halogen to obtain positive charge to be an electrophile?
Introduction
As halogen molecule, for example Br2, approaches a double bond of the alkene, electrons in the double bond repel electrons in bromine molecule causing polarization of the halogen bond. This creates a dipolar moment in the halogen molecule bond. Heterolytic bond cleavage occurs and one of the halogens obtains positive charge and reacts as an electrophile. The reaction of the addition is not regioselective but stereoselective.Stereochemistry of this addition can be explained by the mechanism of the reaction.In the first step electrophilic halogen with a positive charge approaches the double carbon bond and 2 p orbitals of the halogen, bond with two carbon atoms and create a cyclic ion with a halogen as the intermediate step. In the second step, halogen with the negative charge attacks any of the two carbons in the cyclic ion from the back side of the cycle as in the SN2 reaction. Therefore stereochemistry of the product is vicinial dihalides through anti addition.
\[\ce{R_2C=CR_2 + X_2 \rightarrow R_2CX-CR_2X} \tag{8.2.1}\]
Halogens that are commonly used in this type of the reaction are: \(Br\) and \(Cl\). In thermodynamical terms \(I\) is too slow for this reaction because of the size of its atom, and \(F\) is too vigorous and explosive. Solvents that are used for this type of electrophilic halogenation are inert (e.g., CCl4) can be used in this reaction.
Because halogen with negative charge can attack any carbon from the opposite side of the cycle it creates a mixture of steric products.Optically inactive starting material produce optically inactive achiral products (meso) or a racemic mixture.
Electrophilic addition mechanism consists of two steps.
Before constructing the mechanism let us summarize conditions for this reaction. We will use Br2 in our example for halogenation of ethylene.
| Nucleophile | Double bond in alkene |
| Electrophile | Br2, Cl2 |
| Regiochemistry | not relevant |
| Stereochemistry | ANTI |
Step 1: In the first step of the addition the Br-Br bond polarizes, heterolytic cleavage occurs and Br with the positive charge forms a intermediate cycle with the double bond.
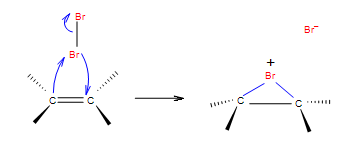
Step 2: In the second step, bromide anion attacks any carbon of the bridged bromonium ion from the back side of the cycle. Cycle opens up and two halogens are in the position anti.
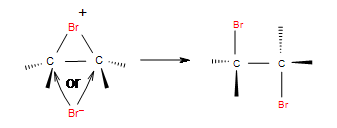
Summary
Hallogens can act as electrophiles due to polarizability of their covalent bond.Addition of halogens is stereospecific and produces vicinial dihalides with anti addition.Cis starting material will give mixture of enantiomers and trans produces a meso compound.
References
- Vollhard,K.Peter C., and Neil E.Schore.Organic Chemistry:Structure and Function.New Yourk: W.H.Freeman and Company 2007
- Chemestry-A Europian Journal 9 (2003) :1036-1044
Exercises
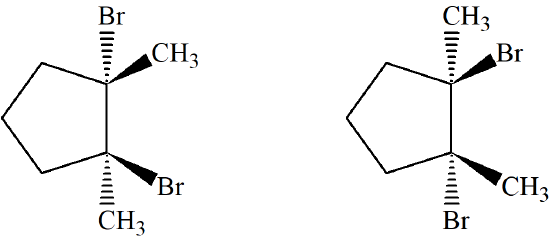
Predict the product of 1,2-dimethylcyclpentene reacting with Br2 with proper stereochemistry.
- Answer
-
Predict the 4 products for 1,2-dimethylcyclpentene reacting with HCl (Section 7.8), give the proper stereochemistry. What is the relationship between the products?
- Answer
-
_Ans.png?revision=1&size=bestfit&width=571&height=222)
In each case HCl, can add on opposite sides of the double bond (anti) on different carbons or on the same side of the double bond (syn) on different carbons. In each case (anti or syn stereochemsitry) the pairs formed are enantiomers of each other.
Predict the product and give the proper stereochemistry.
_Qu.png?revision=1&size=bestfit&width=242&height=81)
- Answer
-
_Sol.png?revision=1&size=bestfit&width=143&height=122)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Jim Clark (Chemguide.co.uk)

