3.1: Functional Groups
- Page ID
- 67067
Objectives
After completing this section, you should be able to
- explain why the properties of a given organic compound are largely dependent on the functional group or groups present in the compound.
- identify the functional groups present in each of the following compound types: alkenes, alkynes, arenes, (alkyl and aryl) halides, alcohols, ethers, aldehydes, ketones, esters, carboxylic acids, (carboxylic) acid chlorides, amides, amines, nitriles, nitro compounds, sulfides and sulfoxides.
- identify the functional groups present in an organic compound, given its structure.
- Given the structure of an organic compound containing a single functional group, identify which of the compound types listed under Objective 2, above, it belongs to.
- draw the structure of a simple example of each of the compound types listed in Objective 2.
Make certain that you can define, and use in context, the key term below.
- functional group
The concept of functional groups is a very important one. We expect that you will need to refer back to tables at the end of Section 3.1 quite frequently at first, as it is not really feasible to learn the names and structures of all the functional groups and compound types at one sitting. Gradually they will become familiar, and eventually you will recognize them automatically.
Functional groups are atoms or small groups of atoms (two to four) that exhibit a characteristic reactivity. A particular functional group will almost always display its characteristic chemical behavior when it is present in a compound. Because of their importance in understanding organic chemistry, functional groups have characteristic names that often carry over in the naming of individual compounds incorporating specific groups
As we progress in our study of organic chemistry, it will become extremely important to be able to quickly recognize the most common functional groups, because they are the key structural elements that define how organic molecules react. For now, we will only worry about drawing and recognizing each functional group, as depicted by Lewis and line structures. Much of the remainder of your study of organic chemistry will be taken up with learning about how the different functional groups tend to behave in organic reactions.
Hydrocarbons and halides
We have already seen some examples of very common functional groups: ethene, for example, contains a carbon-carbon double bond. This double bond is referred to, in the functional group terminology, as an alkene.
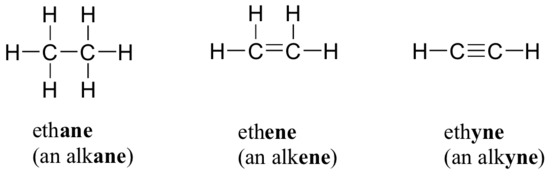
The carbon-carbon triple bond in ethyne is the simplest example of an alkyne function group.
What about ethane? All we see in this molecule is carbon-hydrogen and carbon-carbon single bonds, so in a sense we can think of ethane as lacking a functional group entirely. However, we do have a general name for this ‘default’ carbon bonding pattern: molecules or parts of molecules containing only carbon-hydrogen and carbon-carbon single bonds are referred to as alkanes. If the carbon of an alkane is bonded to a halogen, the group is now referred to as a haloalkane (fluoroalkane, chloroalkane, etc.). Chloroform, CHCl3, is an example of a simple haloalkane.
Alcohols and Thiols
We have already seen the simplest possible example of an alcohol functional group in methanol. In the alcohol functional group, a carbon is single-bonded to an OH group (this OH group, by itself, is referred to as a hydroxyl). If the central carbon in an alcohol is bonded to only one other carbon, we call the group a primary alcohol. In secondary alcohols and tertiary alcohols, the central carbon is bonded to two and three carbons, respectively. Methanol, of course, is in class by itself in this respect.

The sulfur analog of an alcohol is called a thiol (the prefix thio, derived from the Greek, refers to sulfur).

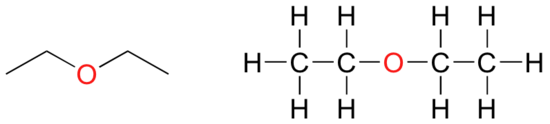
In an ether functional group, a central oxygen is bonded to two carbons. Below are the line and Lewis structures of diethyl ether, a common laboratory solvent and also one of the first medical anaesthesia agents.
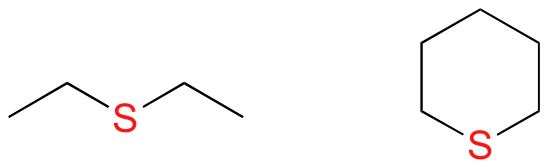
In sulfides, the oxygen atom of an ether has been replaced by a sulfur atom.
Amines and Phosphates
Ammonia is the simplest example of a functional group called amines. Just as there are primary, secondary, and tertiary alcohols, there are primary, secondary, and tertiary amines.
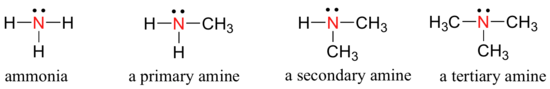
One of the most important properties of amines is that they are basic, and are readily protonated to form ammonium cations.

Phosphorus is a very important element in biological organic chemistry, and is found as the central atom in the phosphate group. Many biological organic molecules contain phosphate, diphosphate, and triphosphate groups, which are linked to a carbon atom by the phosphate ester functionality.
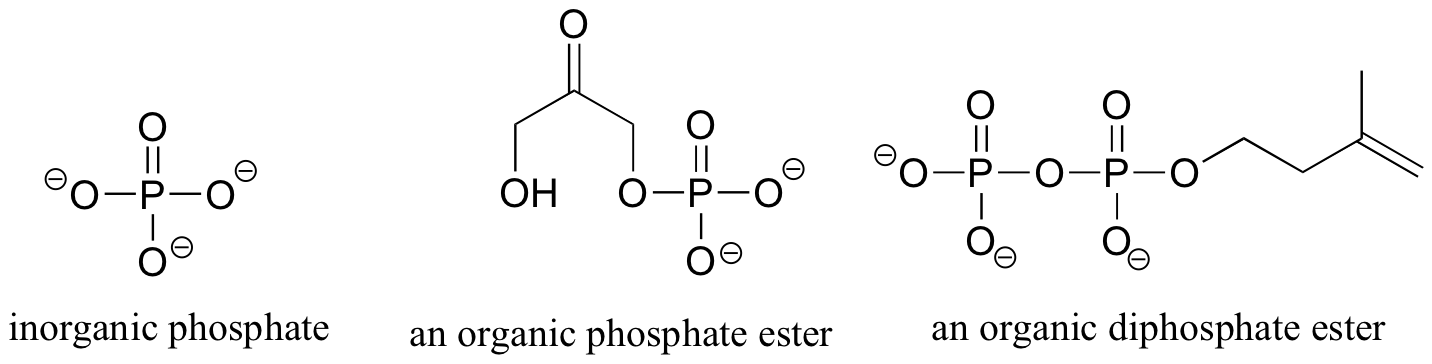
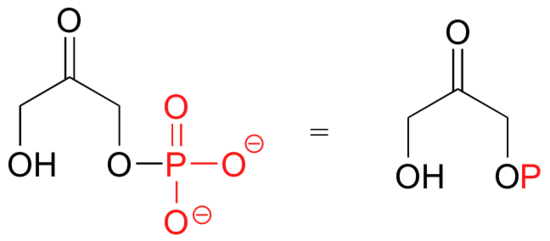
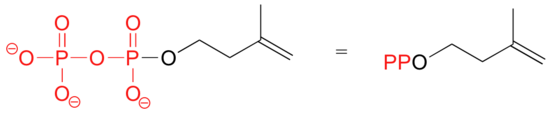
Because phosphates are so abundant in biological organic chemistry, it is convenient to depict them with the abbreviation 'P'. Notice that this 'P' abbreviation includes the oxygen atoms and negative charges associated with the phosphate groups.
Carbonyl Containing Functional Groups
Aldehydes and Ketones
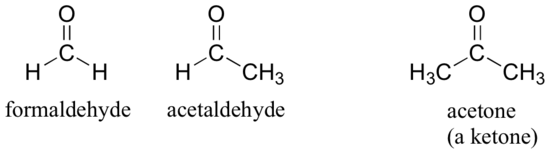
There are a number of functional groups that contain a carbon-oxygen double bond, which is commonly referred to as a carbonyl. Ketones and aldehydes are two closely related carbonyl-based functional groups that react in very similar ways. In a ketone, the carbon atom of a carbonyl is bonded to two other carbons. In an aldehyde, the carbonyl carbon is bonded on one side to a hydrogen, and on the other side to a carbon. The exception to this definition is formaldehyde, in which the carbonyl carbon has bonds to two hydrogens.
Molecules with carbon-nitrogen double bonds are called imines, or Schiff bases.

Carboxylic acids and acid derivatives
If a carbonyl carbon is bonded on one side to a carbon (or hydrogen) and on the other side to a heteroatom (in organic chemistry, this term generally refers to oxygen, nitrogen, sulfur, or one of the halogens), the functional group is considered to be one of the ‘carboxylic acid derivatives’, a designation that describes a grouping of several functional groups. The eponymous member of this grouping is the carboxylic acid functional group, in which the carbonyl is bonded to a hydroxyl (OH) group.
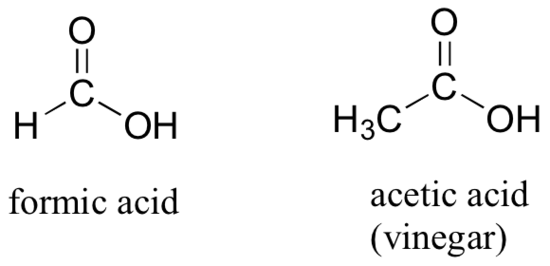
As the name implies, carboxylic acids are acidic, meaning that they are readily deprotonated to form the conjugate base form, called a carboxylate (much more about carboxylic acids in the acid-base chapter!).
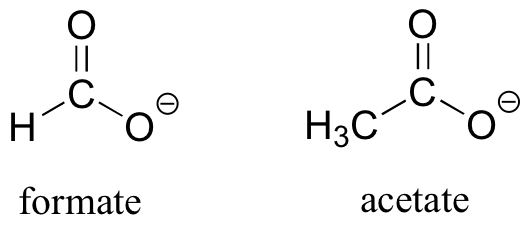
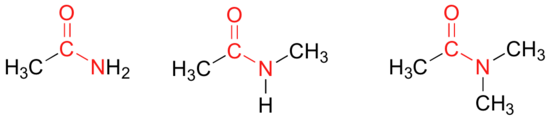
In amides, the carbonyl carbon is bonded to a nitrogen. The nitrogen in an amide can be bonded either to hydrogens, to carbons, or to both. Another way of thinking of an amide is that it is a carbonyl bonded to an amine.
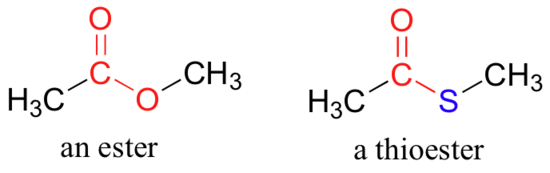
In esters, the carbonyl carbon is bonded to an oxygen which is itself bonded to another carbon. Another way of thinking of an ester is that it is a carbonyl bonded to an alcohol. Thioesters are similar to esters, except a sulfur is in place of the oxygen.
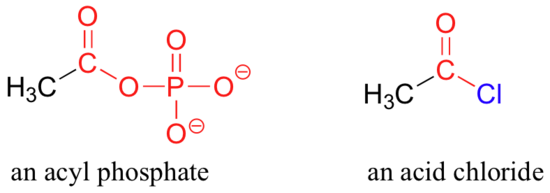
In an acyl phosphate, the carbonyl carbon is bonded to the oxygen of a phosphate, and in an acid chloride, the carbonyl carbon is bonded to a chlorine.
Finally, in a nitrile group, a carbon is triple-bonded to a nitrogen. Nitriles are also often referred to as cyano groups.

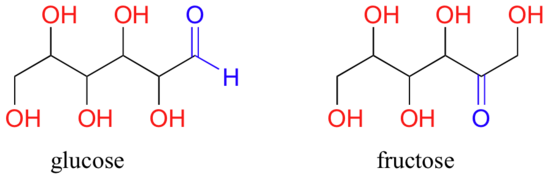
A single compound often contains several functional groups. The six-carbon sugar molecules glucose and fructose, for example, contain aldehyde and ketone groups, respectively, and both contain five alcohol groups (a compound with several alcohol groups is often referred to as a ‘polyol’).
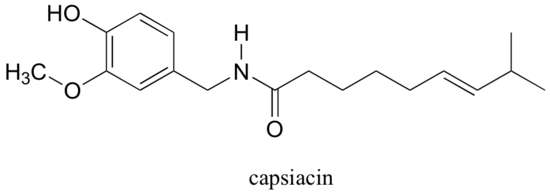
Capsaicin, the compound responsible for the heat in hot peppers, contains phenol, ether, amide, and alkene functional groups.
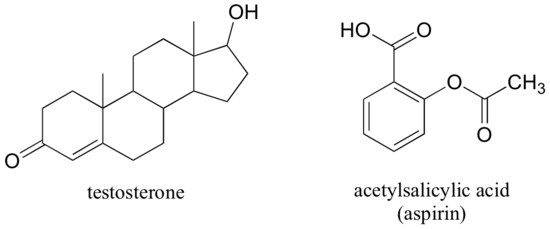
The male sex hormone testosterone contains ketone, alkene, and secondary alcohol groups, while acetylsalicylic acid (aspirin) contains aromatic, carboxylic acid, and ester groups.
While not in any way a complete list, this section has covered most of the important functional groups that we will encounter in biological and laboratory organic chemistry. The table on the inside back cover provides a summary of all of the groups listed in this section, plus a few more that will be introduced later in the text.
Identify the functional groups in the following organic compounds. State whether alcohols and amines are primary, secondary, or tertiary.
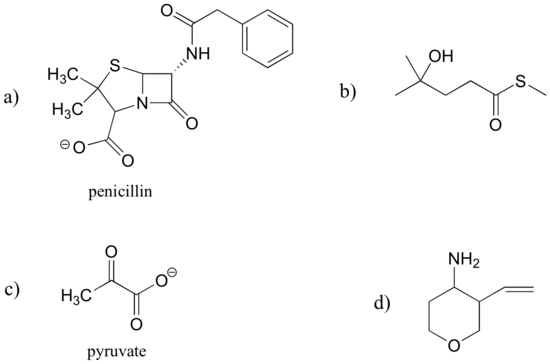
- Answer
-
a) carboxylate, sulfide, aromatic, two amide groups (one of which is cyclic)
b) tertiary alcohol, thioester
c) carboxylate, ketone
d) ether, primary amine, alkene
Functional Group Tables
Exclusively Carbon Functional Groups
| Group Formula | Class Name | Specific Example | IUPAC Name | Common Name |
|---|---|---|---|---|
 |
Alkene | H2C=CH2 | Ethene | Ethylene |
| Alkyne | HC≡CH | Ethyne | Acetylene | |
| Arene | C6H6 | Benzene | Benzene |
Functional Groups with Single Bonds to Heteroatoms
| Group Formula | Class Name | Specific Example | IUPAC Name | Common Name |
|---|---|---|---|---|
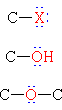 |
Halide | H3C-I | Iodomethane | Methyl iodide |
| Alcohol | CH3CH2OH | Ethanol | Ethyl alcohol | |
| Ether | CH3CH2OCH2CH3 | Diethyl ether | Ether | |
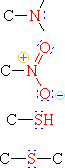 |
Amine | H3C-NH2 | Aminomethane | Methylamine |
| Nitro Compound | H3C-NO2 | Nitromethane | ||
| Thiol | H3C-SH | Methanethiol | Methyl mercaptan | |
| Sulfide | H3C-S-CH3 | Dimethyl sulfid |
Functional Groups with Multiple Bonds to Heteroatoms
| Group Formula | Class Name | Specific Example | IUPAC Name | Common Name |
|---|---|---|---|---|
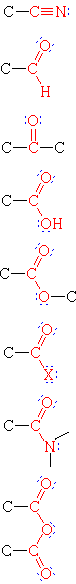 |
Nitrile | H3C-CN | Ethanenitrile | Acetonitrile |
| Aldehyde | H3CCHO | Ethanal | Acetaldehyde | |
| Ketone | H3CCOCH3 | Propanone | Acetone | |
| Carboxylic Acid | H3CCO2H | Ethanoic Acid | Acetic acid | |
| Ester | H3CCO2CH2CH3 | Ethyl ethanoate | Ethyl acetate | |
| Acid Halide | H3CCOCl | Ethanoyl chloride | Acetyl chloride | |
| Amide | H3CCON(CH3)2 | N,N-Dimethylethanamide | N,N-Dimethylacetamide | |
| Acid Anhydride | (H3CCO)2O | Ethanoic anhydride | Acetic anhydride |
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

