13.2: Evaporation and Condensation
- Page ID
- 289446
⚙️ Learning Objectives
- Explain the processes of vaporization, evaporation, and condensation.
Outside the abandoned barracks shown in Figure \(\PageIndex{1}\) below are devices known as a "swamp coolers". This piece of equipment traces its origin back to the ancient Egyptians who hung wet blankets across the doors of their homes. As the warm air passed through the blankets, water would evaporate and cool the air. The royalty went one step further and had servants fan wet cloths over jugs of water to get more evaporation and cooling.

The origin of the term "swamp cooler" is not known – they certainly don't work in a swamp. The best conditions for cooling with a swamp cooler include a high temperature (over 80°F) and a low humidity (preferably less than 30%). These coolers work well in desert areas, but don't provide any cooling in the humid areas of the country.
Evaporation
A puddle of water left undisturbed eventually disappears. The liquid molecules escape into the gas phase, becoming water vapor. Vaporization is the process by which a liquid is converted to a gas. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If the water is instead kept in a closed container, the water vapor molecules do not have a chance to escape into the surroundings and so the water level does not change. As some water molecules become vapor, an equal number of water vapor molecules condense back into the liquid state. Condensation is the change of state from a gas to a liquid. The process where the rate of evaporation of a substance is the same as the rate of condensation in a closed container is called a dynamic equilibrium.
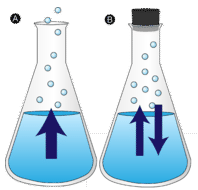
A given liquid sample will have molecules with a wide range of kinetic energies. In order for a liquid molecule to escape into the gaseous state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. Liquid molecules that have this certain threshold kinetic energy escape the surface and become vapor. As a result, the liquid molecules that remain now have lower kinetic energy. As evaporation occurs, the temperature of the remaining liquid decreases. You have observed the effects of evaporative cooling. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body.
A given liquid will evaporate more quickly when it is heated. This is because the heating process results in a greater fraction of the liquid's molecules having the necessary kinetic energy to escape the surface of the liquid. The figure below shows the kinetic energy distribution of liquid molecules at two temperatures. The numbers of molecules that have the required kinetic energy to evaporate are shown in the shaded area under the curve at the right. The higher temperature liquid (T2) has more molecules that are capable of escaping into the vapor phase than the lower temperature liquid (T1).

Boiling
As a liquid is heated, the average kinetic energy of its particles increases. The rate of evaporation increases as more and more molecules are able to escape the liquid's surface into the vapor phase. Eventually a point is reached when the molecules all throughout the liquid have enough kinetic energy to vaporize. At this point the liquid begins to boil. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The vapor pressure is the pressure exerted by the molecules that escape from the surface of the liquid and increases with an increase in temperature. The figure below illustrates the boiling of liquid.
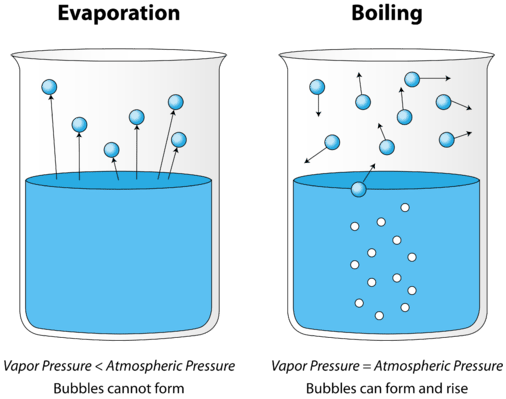
In the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. On the right, the temperature has been increased until bubbles begin to form in the body of the liquid. When the vapor pressure inside the bubble is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. The temperature at which this process occurs is the boiling point of the liquid.
The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. The normal boiling point is a constant because it is defined relative to the standard atmospheric pressure of 760 mm Hg (or 1 atm or 101.325 kPa).
At 29,029 feet (8848 m), Mount Everest in the Himalayan range on the border between China and Nepal is the highest point on the earth. Its altitude presents many practical problems to climbers. The oxygen content of the air is much lower than at sea level, making it necessary to bring oxygen tanks along (although a few climbers have reached the peak without oxygen). One other problem is that of boiling water for cooking food. Although water boils at 100°C at sea level, the boiling point on top of Mount Everest is only about 70°C. This difference makes it very difficult to get a decent cup of tea (which definitely frustrated some of the British climbers).
Is it possible to boil water without heating? Check out the videos below to see how!
✅ Example \(\PageIndex{1}\)
What are two ways to cause water to boil?
Solution
Since the boiling point is defined as the temperature at which the vapor pressure of a liquid is equal to the external pressure, it is is possible to boil a liquid either by increasing its temperature or by reducing the pressure above the liquid.
✏️ Exercise \(\PageIndex{1}\)
- What is the name given to the process of a liquid being converted to a gas when the temperature of the liquid is below its boiling point?
- What is the name of the phase change given to a gas or vapor being converted to a liquid?
- Answer A
- evaporation
- Answer B
- condensation
Summary
- The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure.
- As the altitude increases, the boiling point decreases.
- Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid.
- Condensation is the change of state from a gas to a liquid.
- As the temperature increases, the rate of evaporation increases.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Melissa Alviar-Agnew, Henry Agnew, and Lance S. Lund (Anoka-Ramsey Community College). Original source: https://www.ck12.org/c/chemistry/.



