The Effect of a Catalyst on Rate of Reaction
- Page ID
- 3823
This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the Maxwell-Boltzmann distribution of molecular energies in a gas. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. When the reaction has finished, the mass of catalyst is the same as at the beginning. Several examples of catalyzed reactions and their respective catalysts are given below:
| reaction | catalyst |
|---|---|
| Decomposition of hydrogen peroxide | manganese(IV) oxide, MnO2 |
| Nitration of benzene | concentrated sulfuric acid |
| Manufacture of ammonia by the Haber Process | iron |
| Conversion of SO2 into SO3 during the Contact Process to make sulfuric acid | vanadium(V) oxide, V2O5 |
| Hydrogenation of a C=C double bond | nickel |
The importance of activation energy
Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The position of activation energy can be determined from a on a Maxwell-Boltzmann distribution:
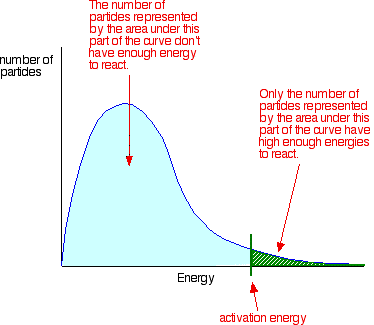
Only those particles represented by the area to the right of the activation energy will react when they collide. The majority do not have enough energy, and will simply bounce apart.
To increase the rate of a reaction, the number of successful collisions must be increased. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. In other words, to move the activation energy to the left on the graph:
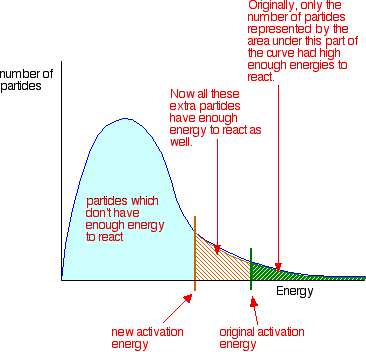
Adding a catalyst has this effect on activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. This is illustrated on the following energy profile:
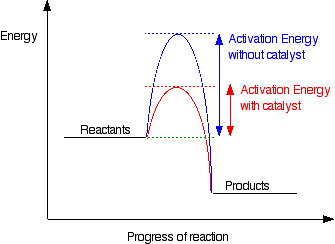
Care must be taken when discussing how a catalyst operates. A catalyst provides an alternative route for the reaction with a lower activation energy. It does not "lower the activation energy of the reaction". There is a subtle difference between the two statements that is easily illustrated with a simple analogy. Suppose there is a mountain between two valleys such that the only way for people to get from one valley to the other is over the mountain. Only the most active people will manage to get from one valley to the other.
Now suppose a tunnel is cut through the mountain. Many more people will now manage to get from one valley to the other by this easier route. It could be said that the tunnel route has a lower activation energy than going over the mountain, but the mountain itself is not lowered. The tunnel has provided an alternative route but has not lowered the original one. The original mountain is still there, and some people still choose to climb it. In chemical terms, if particles collide with enough energy they can still react in exactly the same way as if the catalyst was not there; it is simply that the majority of particles will react via the easier catalyzed route.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


