13.5: Pi Bonding with p Orbitals
- Page ID
- 192605
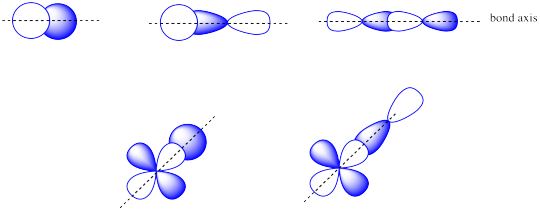
Earlier, we saw that p orbitals that lie along the same axis can interact to form bonds.
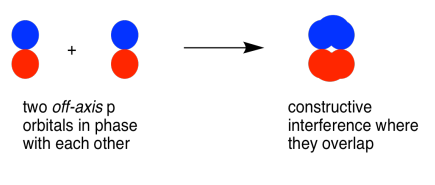
Parallel, but not collinear, p orbitals can also interact with each other. They would approach each other side by side, above and below the bond axis between the two atoms. They can be close enough to each other to overlap, although they do not overlap as strongly as orbitals lying along the bond axis. They can make an in-phase combination, as shown below.
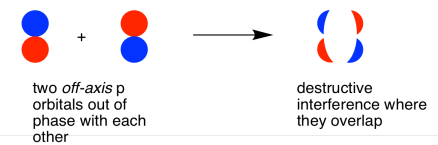
They could also make an out-of-phase combination, as shown below.
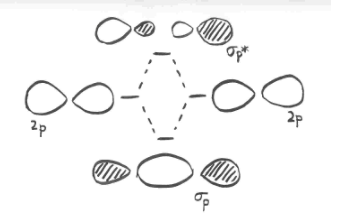
- parallel p orbitals can overlap to produce bonding and antibonding combinations.
- the resulting orbitals contain nodes along the bond axis.
- the electron density is found above and below the bond axis.
- this is called a p (pi) bond.
The illustration above is for one set of p orbitals that are orthogonal to the bond axis. The second picture shows the result of the constructive (or destructive) interference. A similar picture could be shown for the other set of p orbitals.
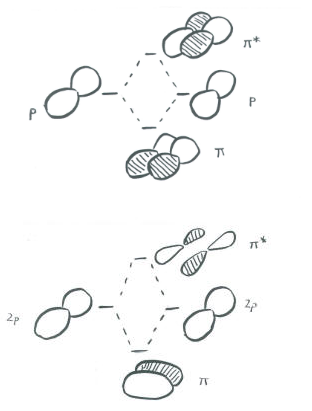
In a main group diatomic species like dinitrogen, one p orbital lying along the bond axis can engage in s bonding. The two p orbitals orthogonal to the bond axis can engage in p bonding. There will be both bonding and antibonding combinations.
Just as the sigma-bonding orbitals display progressively shorter wavelengths along the bonding axis as they go to higher energy, so do the pi bonding orbitals. In other words, there are more nodes in the higher-energy orbitals than in the lower-energy ones.
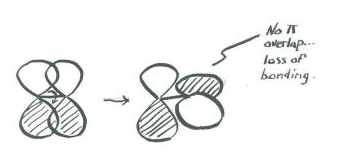
An important consequence of the spatial distribution or "shape" of a p orbital is that it is not symmetric with respect to the bond axis. A s orbital is not affected when the atom at one end of the bond is rotated with respect to the other. A p orbital is affected by rotation. If one atom turns with respect to the other, the p orbital would have to stretch to maintain the connection. The orbitals would not be able to overlap, so the connection between the atoms would be lost.
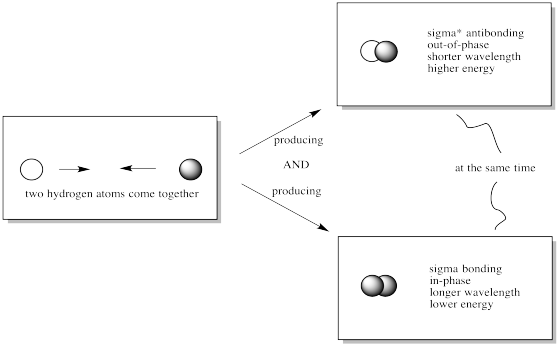
Exercise \(\PageIndex{1}\)
When the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule, which molecular orbitals are formed?
Draw a picture.
- Answer
-
When 2 atomic orbitals are combined, 2 molecular orbitals are formed: one in-phase bonding orbital and one out-of-phase antibonding orbital.
Exercise \(\PageIndex{2}\)
In-phase combinations of atomic orbitals give ______________ orbitals.
Draw a picture
- Answer
-
AIn-phase combinations of atomic orbitals give bonding orbitals.
Exercise \(\PageIndex{3}\)
Out-of-phase combinations of atomic orbitals give ______________ orbitals.
Draw a picture.
- Answer
-
Out-of-phase combinations of atomic orbitals give antibonding orbitals.
Exercise \(\PageIndex{4}\)
The combinations of ______________ atomic orbitals leads to σ orbitals.
Draw pictures.
- Answer
-
The combinations of s + s OR s + p OR p + p OR s + d OR p + d atomic orbitals can lead to σ orbitals.
Exercise \(\PageIndex{5}\)
The combinations of ______________ atomic orbitals leads to π orbitals.
Draw pictures.
- Answer
-
The combinations of side by side p + p or p + d atomic orbitals leads to π orbitals.

Exercise \(\PageIndex{6}\)
Which molecular orbital is typically the highest in energy?
a. p
b. σ
c. π*
d. π
e. σ*
- Answer
-
e) σ*
Exercise \(\PageIndex{7}\)
Why can 1s orbital not interact with a 2s orbital?
Hint: Why is a Li2O bond stronger than a K2O bond?
- Answer
-
Li+ and O2- are more similar in size than K+ and O2-, so the bond between Li+ and O2- is stronger.
The energy difference between the 1s orbitals and 2s orbitals is too large, so they cannot interact. In order for orbitals to interact, the orbitals need to have the same symmetry, be in the same plane, and be similar in energy.
Exercise \(\PageIndex{8}\)
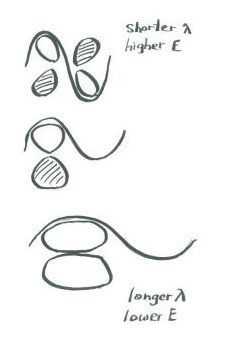
Add a few words to explain the ideas conveyed in these drawings.
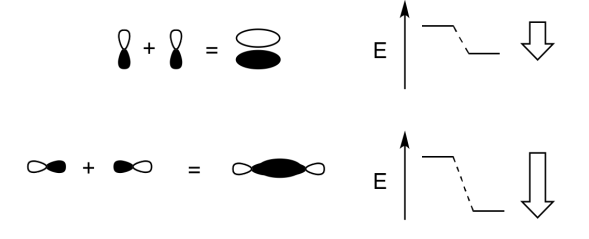
- Answer
-
When two parallel p orbitals combine out-of-phase, destructive intereference occurs.
There is a node between the atoms.
The energy of the electrons increases.
When two parallel p orbitals combine in-phase, constructive interference occurs.
There is no node between the atoms; the electrons are found above and below the axis connecting the atoms.
The energy of the electrons decreases.


