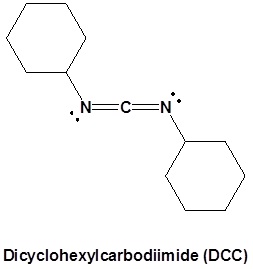
Conversion of Carboxylic acids to amides using DCC as an activating agent
- Page ID
- 5534
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
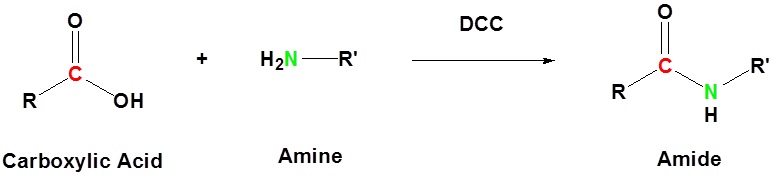
The direct conversion of a carboxylic acid to an amide is difficult because amines are basic and tend to convert carboxylic acids to their highly unreactive carboxylates. In this reaction the carboxylic acid adds to the DCC molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. DCC induced coupling to form an amide linkage is an important reaction in the synthesis of peptides.
Basic reaction
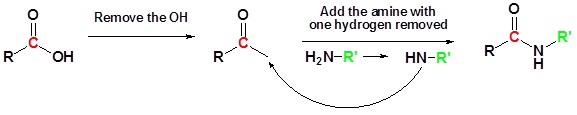
Going from reactants to products simplified
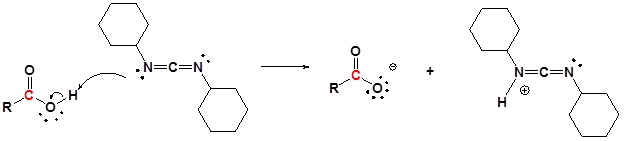
Mechanism
1) Deprotonation
2) Nucleophilic attack by the carboxylate

3) Nucleophilic attack by the amine
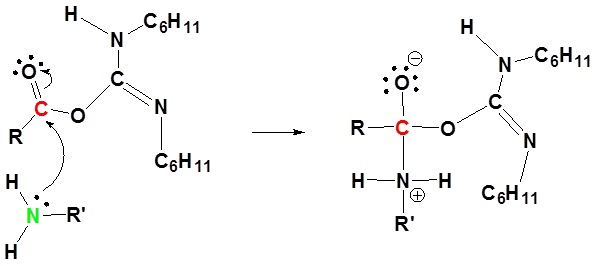
4) Proton transfer
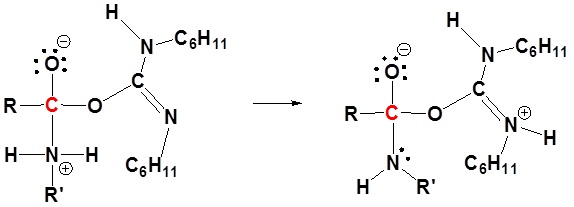
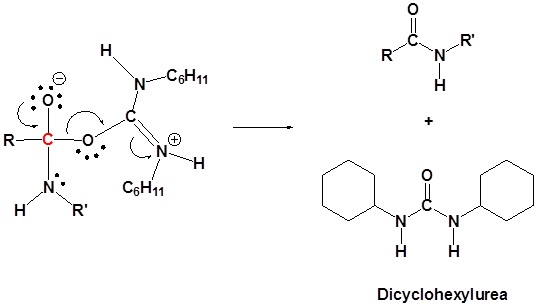
5) Leaving group removal
Contributors
Prof. Steven Farmer (Sonoma State University)

