Interhalogens
- Page ID
- 11265
The halogens react with each other to form interhalogen compounds. The general formula of most interhalogen compounds is XYn, where n = 1, 3, 5 or 7, and X is the less electronegative of the two halogens. The compounds which are formed by the union of two different halogens are called interhalogen compounds. There are never more than two types of halogen atoms in an interhalogen molecule. These are of four general types:
- AX- type : ClF, BrF, BrCl, ICl, IBr,
- AX3-type: ClF3, BrF3, (ICl3)2,
- AX5-type: ClF5, BrF5, IF5,
- AX7-type: IF7.
The interhalogen compounds of type AX and AX3 are formed between halogens having a very low electronegativity difference (e.g., ClF, ClF3). The interhalogen compounds of type AX5 and AX7 are formed by larger atoms having a low electronegativity with the smaller atoms having a high electronegativity. This is because it is possible to fit a greater number of smaller atoms around a larger one (e.g., BrF5, IF7).
Interhalogens are all prone to hydrolysis and ionize to give rise to polyatomic ions. The interhalogens are generally more reactive than halogens except for F. This is because A-X bonds in interhalogens are weaker than the X-X bonds in dihalogen molecules. Reactions of interhalogens are similar to those of halogens. Hydrolysis of interhalogen compounds gives a halogen acid and an oxy-acid.
To name an interhalogen compound, the less electronegative element is placed on to the left in formulas and naming is straightforward.
Properties
Some properties of interhalogen compounds are listed below. They are all prepared by direct combination of the elements, although since in some cases more than one product is possible, the conditions may be varied by altering the temperature and relative proportions. For example, under the same conditions, difluorine reacts with dichlorine to give ClF, with dibromine to give BrF3, but with diiodine to give IF5.
| Compound | ClF | BrF | BrCl | ICl | IBr | ClF3 | BrF3 | IF3 | I2Cl6 | ClF5 | BrF5 | IF5 | IF7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance at 298K | Colorless gas | Pale brown gas | impure | Red solid | Black solid | Colorless gas | Yellow liquid | Yellow solid | Orange solid | Colorless gas | Colorless liquid | Colorless liquid | Colorless gas |
| Stereochemistry | linear | linear | linear | linear | linear | T-shaped | T-shaped | T-shaped | planar | square-based pyramid | square-based pyramid | square-based pyramid | pentagonal bipyramid |
| Melting point /K | 117 | ~240 | dissoc. | 300(a) | 313 | 197 | 282 | 245 (dec) | 337 (sub) | 170 | 212.5 | 282.5 | 278 (sub) |
| Boiling point /K | 173 | ~293 | ~278 | ~373 | 389 | 285 | 399 | - | - | 260 | 314 | 373 | - |
| ΔfH°(298 K) /kJ mol-1 | -50.3 | -58.5 | 14.6 | -23.8 | -10.5 | -163.2 | -300.8 | ~-500 | -89.3 | -255 | -458.6 | -864.8 | -962 |
| Dipole moment for gas-phase molecule /D | 0.89 | 1.42 | 0.52 | 1.24 | 0.73 | 0.6 | 1.19 | - | 0 | - | 1.51 | 2.18 | 0 |
| Bond distances in gas-phase molecules except for IF3 and I2Cl6 / pm | 163 | 176 | 214 | 232 | 248.5 | 160 (eq), 170 (ax) | 172 (eq), 181 (ax) | 187 (eq), 198 (ax) | 238 (terminal) 268 (bridge) | 172 (basal), 162 (apical) | 178 (basal), 168 (apical) | 187 (basal), 185 (apical) | 186 (eq), 179 (ax) |
Structures
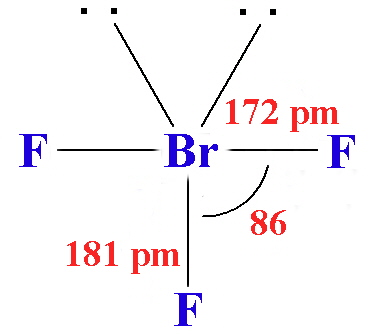
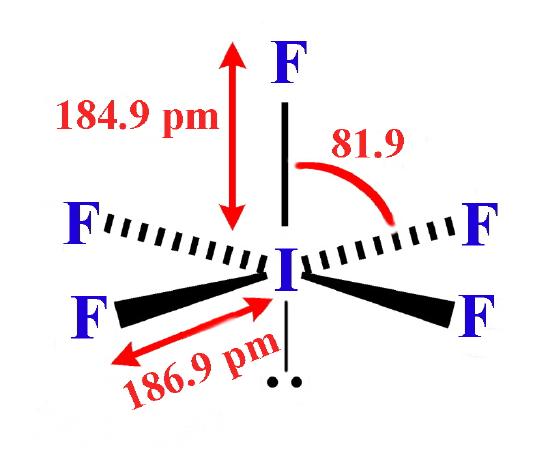
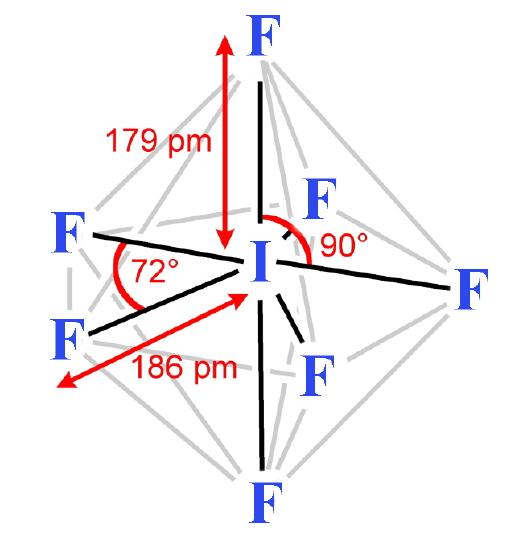
The structures found for the various interhalogens conform to what would be expected based on the VSEPR model. For XY3 the shape can be described as T-shaped with 2 lone pairs sitting in two of the equatorial positions of a trigonal bipyramid. For XY5 the shape is a square pyramid with the unpaired electrons sitting in an axial position of an octahedron, and XY7 is a pentagonal bipyramid.
XY diatomic interhalogens
The interhalogens with formula XY have physical properties intermediate between those of the two parent halogens. The covalent bond between the two atoms has some ionic character, with the larger element, X, becoming oxidized and having a partial positive charge. Most combinations of F, Cl, Br and I are known, but not all are stable.
- Chlorine monofluoride (ClF), the lightest interhalogen, is a colorless gas with a boiling point of 173 °K.
- Bromine monofluoride (BrF) has not been obtained in the pure form - it dissociates into the trifluoride and free bromine. Similarly, iodine monofluoride is unstable - iodine reacts with fluorine to form a pentafluoride.
- Iodine monofluoride (IF) is unstable and disproportionates rapidly and irreversibly at room temperature: \[\ce{5IF → 2I2 + IF5} \nonumber\]. However, its molecular properties have been determined by spectroscopy: the iodine-fluorine distance is 190.9 pm and the I-F bond dissociation energy is around 277 kJ mol-1. ΔHf° = -95.4 kJ mol-1 and ΔGf° = -117.6 kJ mol-1, both at 298 K.
\(\ce{IF}\) can be generated by the following reactions:
I2 + F2 → 2IF at -45 °C in CCl3F;
I2 + IF3 → 3IF at -78 °C in CCl3F;
I2 + AgF → IF + AgI at 0 °C. - Bromine monochloride (BrCl) is an unstable red-brown gas with a boiling point of 5 °C.
- Iodine monochloride (ICl) consists of red transparent crystals which melt at 27.2 °C to form a choking brownish liquid (similar in appearance and weight to bromine). It reacts with HCl to form the strong acid HICl2. The crystal structure of iodine monochloride consists of puckered zig-zag chains, with strong interactions between the chains.
- Iodine monobromide (IBr) is made by direct combination of the elements to form a dark red crystalline solid. It melts at 42 °C and boils at 116 °C to form a partially dissociated vapor.
XY3 interhalogens
- Chlorine trifluoride (ClF3) is a colorless gas that condenses to a green liquid and freezes to a white solid. It is made by reacting chlorine with an excess of fluorine at 250° C in a nickel tube. It reacts more violently than fluorine, often explosively. The molecule is planar and T-shaped.
- Bromine trifluoride (BrF3) is a yellow-green liquid that conducts electricity - it ionizes to form [BrF2+] + [BrF4-]. It reacts with many metals and metal oxides to form similar ionized entities; with some others it forms the metal fluoride plus free bromine and oxygen. It is used in organic chemistry as a fluorinating agent. It has the same molecular shape as chlorine trifluoride.
- Iodine trifluoride (IF3) is a yellow solid which decomposes above -28 °C. It can be synthesized from the elements, but care must be taken to avoid the formation of IF5. F2 attacks I2 to yield IF3 at -45 °C in CCl3F. Alternatively, at low temperatures, the fluorination reaction I2 + 3XeF2 → 2IF3 + 3Xe can be used. Not much is known about iodine trifluoride as it is so unstable.
- Iodine trichloride (ICl3) forms lemon yellow crystals which can be melted under pressure to a brown liquid. It can be made from the elements at low temperature, or from iodine pentoxide and hydrogen chloride. It reacts with many metal chlorides to form tetrachloriodides, and hydrolyses in water. The molecule is a planar dimer, with each iodine atom surrounded by four chlorine atoms. In the melt it is conductive, which may indicate dissociation: \[\ce{I_2Cl_6 → ICl_2^{+} + ICl_4^{-}} \nonumber \]
Chlorine trifluoride, ClF3, was first reported in 1931; it is primarily used for the manufacture of uranium hexafluoride, UF6, as part of nuclear fuel processing and reprocessing by the reaction:
\[\ce{U + 3 ClF_3 → UF_6 + 3 ClF} \nonumber \]
U isotope separation is difficult because the two isotopes have very nearly identical chemical properties, and can only be separated gradually using small mass differences. (235U is only 1.26% lighter than 238U.) A cascade of identical stages produces successively higher concentrations of 235U. Each stage passes a slightly more concentrated product to the next stage and returns a slightly less concentrated residue to the previous stage.
There are currently two generic commercial methods employed internationally for enrichment: gaseous diffusion (referred to as first generation) and gas centrifuge (second generation), which consumes only 6% as much energy as gaseous diffusion. These both make use of the volatility of UF6.
ClF3 has been investigated as a high-performance storable oxidizer in rocket propellant systems. Handling concerns, however, prevented its use.
Hypergolic means explodes on contact with no need for any activator. One observer made the following comment about \(ClF_3\):
"It is, of course, extremely toxic, but that's the least of the problem. It is hypergolic* with every known fuel, and so rapidly hypergolic that no ignition delay has ever been measured. It is also hypergolic with such things as cloth, wood, and test engineers, not to mention asbestos, sand, and water with which it reacts explosively. It can be kept in some of the ordinary structural metals-steel, copper, aluminium, etc.-because of the formation of a thin film of insoluble metal fluoride which protects the bulk of the metal, just as the invisible coat of oxide on aluminium keeps it from burning up in the atmosphere. If, however, this coat is melted or scrubbed off, and has no chance to reform, the operator is confronted with the problem of coping with a metal-fluorine fire. For dealing with this situation, I have always recommended a good pair of running shoes."
It is believed that prior to and during World War II, ClF3 code named N-stoff ("substance N") was being stockpiled in Germany for use as a potential incendiary weapon and poison gas. The plant was captured by the Russians in 1944, but there is no evidence that the gas was actually ever used during the war.
XY5 interhalogens
- Chlorine pentafluoride (ClF5) is a colorless gas, made by reacting chlorine trifluoride with fluorine at high temperatures and high pressures. It reacts violently with water and most metals and nonmetals.
- Bromine pentafluoride (BrF5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200° C. It is physically stable, but reacts violently with water and most metals and nonmetals.
- Iodine pentafluoride (IF5) is a colorless liquid, made by reacting iodine pentoxide with fluorine, or iodine with silver fluoride. It is highly reactive, even slowly with glass. It reacts with elements, oxides and carbon halides. The molecule has the form of a tetragonal pyramid.
- Primary amines react with iodine pentafluoride to form nitriles after hydrolysis with water. \[R-CH_2-NH_2 → R-CN \nonumber \]
XY7 interhalogens
- Iodine heptafluoride (IF7) is a colorless gas. It is made by reacting the pentafluoride with fluorine. IF7 is chemically inert, having no lone pair of electrons in the valency shell; in this it resembles sulfur hexafluoride. The molecule is a pentagonal bipyramid. This compound is the only interhalogen compound possible where the larger atom is carrying seven of the smaller atoms.
- All attempts to form bromine heptafluoride have met with failure; instead, bromine pentafluoride and fluorine gas are produced.
Diatomic Interhalogens (AX)
The interhalogens of form XY have physical properties intermediate between those of the two parent halogens. The covalent bond between the two atoms has some ionic character, the less electronegative element, X, being oxidized and having a partial positive charge. Most combinations of F, Cl, Br and I are known, but not all are stable.
- Chlorine monofluoride (ClF): The lightest interhalogen compound, ClF is a colorless gas with a normal boiling point of -100 °C.
- Bromine monofluoride (BrF): BrF has not been obtained in a pure form; it dissociates into the trifluoride and free bromine.
- Iodine monofluoride (IF): IF is unstable and decomposes at 0 C, disproportionating into elemental iodine and iodine pentafluoride.
- Bromine monochloride (BrCl): A red-brown gas with a boiling point of 5 °C.
- Iodine monochloride (ICl): Red transparent crystals which melt at 27.2 °C to form a choking brownish liquid (similar in appearance and weight to bromine). It reacts with HCl to form the strong acid HICl2. The crystal structure of iodine monochloride consists of puckered zig-zag chains, with strong interactions between the chains.
- Iodine monobromide (IBr): Made by direct combination of the elements to form a dark red crystalline solid. It melts at 42 °C and boils at 116 °C to form a partially dissociated vapor.
Tetra-atomic Interhalogens (AX3)
- Chlorine trifluoride (ClF3) is a colorless gas which condenses to a green liquid and freezes to a white solid. It is made by reacting chlorine with an excess of fluorine at 250 °C in a nickel tube. It reacts more violently than fluorine, often explosively. The molecule is planar and T-shaped. It is used in the manufacture of uranium hexafluoride.
- Bromine trifluoride (BrF3) is a yellow-green liquid which conducts electricity and ionizes to form [BrF2+] + [BrF4-]. It reacts with many metals and metal oxides to form similar ionized entities; with some others it forms the metal fluoride plus free bromine and oxygen. It is used in organic chemistry as a fluorinating agent. It has the same molecular shape as chlorine trifluoride.
- Iodine trifluoride (IF3) is a yellow solid which decomposes above -28 °C. It can be synthesized from the elements, but care must be taken to avoid the formation of IF5. F2 attacks I2 to yield IF3 at -45 °C in CCl3F. Alternatively, at low temperatures, the fluorination reaction I2+ 3XeF2 --> 2IF3 + 3Xe can be used. Not much is known about iodine trifluoride as it is so unstable.
- Iodine trichloride (ICl3) forms lemon yellow crystals which can be melted under pressure to a brown liquid. It can be made from the elements at low temperature, or from iodine pentoxide and hydrogen chloride. It reacts with many metal chlorides to form tetrachloriodides, and hydrolyses in water. The molecule is a planar dimer, with each iodine atom surrounded by four chlorine atoms.
Hexa-atomic Interhalogens (AX5)
- Chlorine pentafluoride (ClF5) is a colorless gas, made by reacting chlorine trifluoride with fluorine at high temperatures and high pressures. It reacts violently with water and most metals and nonmetals.
- Bromine pentafluoride (BrF5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200Å C. It is physically stable, but reacts violently with water and most metals and nonmetals.
- Iodine pentafluoride (IF5) is a colorless liquid, made by reacting iodine pentoxide with fluorine, or iodine with silver fluoride. It is highly reactive, even slowly with glass. It reacts with elements, oxides and carbon halides.The molecule has the form of a tetragonal pyramid.
Octa-atomic interhalogens (AX7)
- Iodine heptafluoride (IF7) is a colourless gas. It is made by reacting the pentafluoride with fluorine. IF7 is chemically inert, having no lone pair of electrons in the valency shell; in this it resembles sulfur hexafluoride. The molecule is a pentagonal bipyramid. This compound is the only interhalogen compound possible where the larger atom is carrying seven of the smaller atoms
- All attempts to form bromine heptafluoride (BrF7) have failed and instead produce bromine pentafluoride (BrF5) gas.
Summary
All interhalogens are volatile at room temperature. All are polar due to differences in their electronegativity. These are usually covalent liquids or gases due to small electronegativity differences among them. Some compounds partially ionize in solution. For example: \[\ce{2 ICl \rightarrow I^{+} + ICl_2^{-}} \nonumber \] Interhalogen compounds are more reactive than normal halogens, except for fluorine.


