Theoretical Background (high school students)
- Page ID
- 60667
The Electromagnetic Spectrum
Electromagnetic radiation, such as light, is composed of particles called photons that travel through space in a wave motion (Fig. 1).
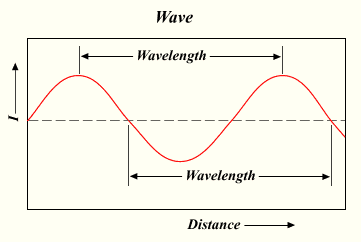
Figure 1: A wave processing, with wavelength depicted. From Wikipedia under Creative Commons License. http://en.wikibooks.org/wiki/File:Wavelength.png
There are many types of electromagnetic radiation. They all, of course, travel at the speed of light, c, but different types of electromagnetic radiation have different frequencies and wavelengths. The wavelength (λ), shown in Figure 1 above, is the distance from one peak of a wave to the next, and is usually measured in meters. The frequency (ν) is the number of waves that pass through a certain point per second, and is measured in s-1, or inverse seconds. The inverse second is also called the Hertz, abbreviated as Hz.
The frequency and the wavelength are inversely proportional to the speed of light, and can be solved for using the equation
λ ν = c
where c = (3.00 x 108 m/s). A particle of electromagnetic radiation with a long wavelength will have a small frequency, meaning that the longer the wave, the more time it will take to pass a certain point and the fewer the number of waves that can pass that point per second.
The energy of a photon can be calculated using the equation
E = hν = hc/λ ,
where h is the Planck constant, 6.626 x 10-34 J s. Photons with high energy have a short wavelength and high frequency. The highest-energy forms of radiation are gamma rays and x-rays. The longest and lowest-energy form of electromagnetic radiation are radio waves. The different types of radiation are pictured in Figure 2 below. Notice how small the portion of the electromagnetic spectrum visible to the human eye is!
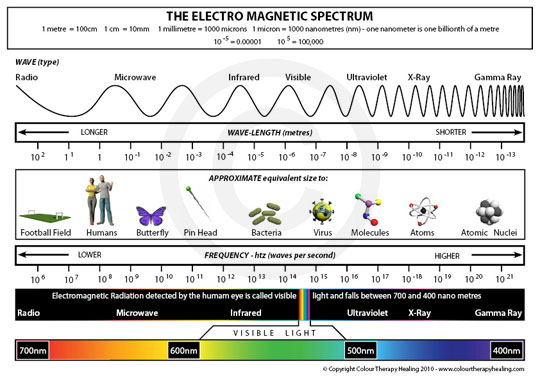
Figure 2: The electromagnetic spectrum, showing relative wavelengths and frequencies, with visible light expanded.
Color and the absorbance of light
As you can see from Figure 2, white light is not one of the colors that are a part of the visible spectrum. What we call 'white' is really a mixture of all of the colors in the spectrum. We can separate white light into the different colors that make it up using a prism(Figure 3) or diffraction gratings (Figure 4).
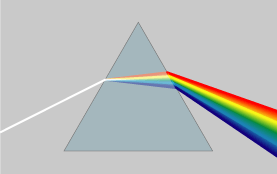
Figure 3: A beam of white light dispersing into its component colors.
A transmission diffraction grating is made up of a transparent material with regularly spaced grooves cut into it so that a beam of light passing through is separated out. You will be using a transmission grating in the camera spectrophotometer.

Figure 4: Dispersion of light in a transmission grating
When white light strikes an object, usually some of the radiation is reflected off of the object and some is absorbed. We see only the reflected light. If you are seeing a red t-shirt, then the dyes that color the t-shirt have reflected all of the red light and have absorbed the yellow, blue, and green light. If the t-shirt is white, that means that all of the light has been reflected off of the t-shirt. If it is black, then almost all of it has been absorbed by the t-shirt, with very little being reflected. This is the reason black clothing is hotter than white clothing on sunny days- all of the energy from the sun is being absorbed by the t-shirt, causing it to heat up.
If instead of shining white light on a solid object, we shine it through a transparent solution, we will see a similar effect. Clear liquids, such as water, are clear because they do not absorb visible light. (Water, however, does absorb other wavelengths of radiation, as you know if you have ever used a microwave to heat water). Colored solutions have color because the solution has absorbed some of the wavelengths of white light and reflected or transmitted the rest of the spectra. Lemonade, for example, is absorbing the red, green, and blue light and allowing the yellow to pass through.
Spectrophotometers are instruments that measure which wavelengths of light have been transmitted and absorbed by solutions, and also tells how much of the light has been absorbed. Dark colored solutions have absorbed more light than a light-colored solution of the same color, and the spectrophotometer can measure and quantify this. The basic parts of our spectrophotometer are:
- A light source
- the sample to be measured
- a diffraction grating to separate the light into the visible spectrum
- a camera to measure the transmitted light
White light from the light source will pass through the sample and some of the light will be absorbed. The remainder of the light will pass through the grating and be separated into the remaining wavelengths, and we will measure the absorbance of the sample using our eyes and, more quantitatively, a camera and the analysis program.
Stray Light
Spectrophotometers work best when the only light in the area is from the light source. Outside light, such as from the classroom lights or sunlight through the window, can make the experiment more difficult. The general term for this undesirable light is stray light. Stray light in our experiment may make it difficult to see the spectrum from the grating and may make the spectrum more diffuse even if it shows up. To decrease the amount of stray light, the experiment should be preformed in a reasonably darkened room.


