Liquid-Liquid Extraction
- Page ID
- 70883
Before examining chromatographic separations, it is useful to consider the separation process in a liquid-liquid extraction. Certain features of this process closely parallel aspects of chromatographic separations. The basic procedure for performing a liquid-liquid extraction is to take two immiscible phases, one of which is usually water and the other of which is usually an organic solvent. The two phases are put into a device called a separatory funnel, and compounds in the system will distribute between the two phases. There are two terms used for describing this distribution, one of which is called the distribution coefficient (DC), the other of which is called the partition coefficient (DM).
The distribution coefficient is the ratio of the concentration of solute in the organic phase over the concentration of solute in the aqueous phase (the V-terms are the volume of the phases). This is essentially an equilibration process whereby we start with the solute in the aqueous phase and allow it to distribute into the organic phase.
\[\mathrm{solute_{aq} = solute_{org}}\]
\[\mathrm{D_C = \dfrac{[solute]_{org}}{[solute]_{aq}} = \dfrac{mol_{org}/V_{org}}{mol_{aq}/V_{aq}} = \dfrac{mol_{org}\times V_{aq}}{mol_{aq}\times V_{org}}}\]
The distribution coefficient represents the equilibrium constant for this process. If our goal is to extract a solute from the aqueous phase into the organic phase, there is one potential problem with using the distribution coefficient as a measure of how well you have accomplished this goal. The problem relates to the relative volumes of the phases. For example, suppose the volume of the organic phase was very small compared to the volume of the aqueous phase. (Imagine using 100 mL of organic solvent relative to a volume of water equal to that in an Olympic-sized swimming pool). You could have a very high concentration of the solute in the organic phase, but if we looked at the amount of solute in the organic phase relative to the amount still in the water, it might only be a small portion of the total solute in the system. Since we really want as much of the solute in the organic phase as possible, this system has not yet achieved that outcome.
The partition coefficient is the ratio of the moles of solute in the two phases, and is a more effective means of measuring whether you have achieved the desired goal. The larger the value of DM, the more of the solute we have extracted or partitioned into the organic phase.
\[\mathrm{D_M = \dfrac{mol_{org}}{mol_{aq}}}\]
Note as well how we can relate DC to DM:
\[\mathrm{D_C = \dfrac{mol_{org}\times V_{aq}}{mol_{aq}\times V_{org}} = D_M\left(\dfrac{V_{aq}}{V_{org}} \right )}\]
From experience you have probably had in your organic chemistry lab, you know that the approach that is often used in liquid-liquid extraction is to add some organic phase, shake the mixture, and remove the organic phase. A fresh portion of the organic phase is then added to remove more of the solute in a second extraction. As we will see shortly, this distribution of a solute between two immiscible phases forms the basis of chromatographic separations as well.
Next we want to examine some general types of extraction procedures that are commonly used. The first is a classic example of an extraction procedure that can be used to separate acids, bases, and neutrals.
An aqueous sample contains a complex mixture of organic compounds, all of which are at trace concentrations. The compounds can be grouped into broad categories of organic acids, organic bases and neutral organics. The desire is to have three solutions at the end, each in methylene chloride, one of which contains only the organic acids, the second contains only the organic bases, and the third contains only the neutrals. Devise an extraction procedure that would allow you to perform this bulk separation of the three categories of organic compounds.
Two things to remember:
- Ionic substances are more soluble in water than in organic solvents.
- Neutral substances are more soluble in organic solvents than in water.
The key to understanding how to do this separation relates to the effect that pH will have on the different categories of compounds.
Neutrals – Whether the pH is acidic or basic, these will remain neutral under all circumstances.
Organic acids – RCOOH
At very acidic pH values (say a pH of around 1) – these are fully protonated and neutral
At basic pH values (say a pH of around 13) – these are fully deprotonated and anionic
Organic bases – R3N
At very acidic pH values (say a pH of around 1) – these are protonated and cationic
At very basic pH values (say a pH of around 13) – these are not protonated and neutral
Step 1: Lower the pH of the water using concentrated hydrochloric acid.
Neutrals – neutral
Acids – neutral
Bases – cationic
Extract with methylene chloride – the neutrals and acids go into the methylene chloride, the bases stay in the water.
Step 2: Remove the water layer from step (1), adjust the pH back to a value of 13 using a concentrated solution of sodium hydroxide, shake against methylene chloride, and we now have a solution of the organic bases in methylene chloride. (Solution 1 – ORGANIC BASES IN METHYLENE CHLORIDE)
Step 3: Take the methylene chloride layer from step (1) and shake this against an aqueous layer with a pH value of 13 (adjusted to that level using a concentrated solution of sodium hydroxide).
Neutrals – neutral
Organic acids – anionic
The neutrals stay in the methylene chloride layer. (Solution 2: NEUTRALS IN METHYLENE CHLORIDE) The acids go into the water layer.
Step 4. Take the water layer from Step (3), lower the pH to a value of 1 using concentrated hydrochloric acid, shake against methylene chloride, and the neutral organic acids are now soluble in the methylene chloride (Solution 3: ORGANIC ACIDS IN METHYLENE CHLORIDE).
Devise a way to solubilize the organic anion shown below in the organic solvent of a two-phase system in which the second phase is water. As a first step to this problem, show what might happen to this compound when added to such a two-phase system.
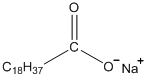
This compound will align itself right along the interface of the two layers. The non-polar C18 group is hydrophobic and will be oriented into the organic phase. The polar carboxylate group is hydrophilic and will be right at the interface with the aqueous phase.
One way to solubilize this anion in the organic phase is to add a cation with similar properties. In other words, if we added an organic cation that has a non-polar R group, this would form an ion pair with the organic anion. The ion pair between the two effectively shields the two charged groups and allows the pair to dissolve in an organic solvent. Two possible organic cations that could be used in this system are cetylpyridinium chloride or tetra-n-butylammonium chloride.

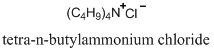
A somewhat similar procedure can often be used to extract metal complexes into an organic phase. Metal salts with inorganic anions (halide, sulfate, phosphate, etc.) are generally water-soluble but not organic-soluble. It is possible to add a relatively hydrophobic ligand to the system. If the ligand complexes with the metal ion, then the metal complex may be organic-soluble. Usually it helps to form a neutral metal complex. Also, remember back to our examination of the effect of pH on the complexation of metal ions with ligands. The extraction efficiency of a metal ion in the presence of a ligand will depend on the pH of the aqueous phase. Adjustment of the pH is often used to alter the selectivity of the extraction, thereby allowing different metal ions to be separated.


