4.32: Modeling the Pi-electrons of Benzene as Particles in a Ring
- Page ID
- 151349
In this exercise benzene's six π electrons will be modeled as particles in a ring or circular corral. Schrödinger's equation in plane polar coordinates and its energy eigenvalues are given below. R is the ring radius and C the ring circumference.
\[ \begin{matrix} \frac{-h^2}{8 \pi^2 m_e} \left( \frac{d^2}{dr^2} \Psi (r) + \frac{1}{r} \frac{d}{dr} \Psi (r) - \frac{L^2}{r^2} \Psi (r) \right) = E \Psi (r) & E_{n,~L} = \frac{ \left( Z_{n,~L} \right)^2 h^2}{8 \pi^2 m_e R^2} = \frac{ \left( Z_{n,~L} \right)^2 n^2}{2 m_e C^2} \end{matrix} \nonumber \]
JL is the Lth order Bessel function, L is the angular momentum quantum number, n is the principle quantum number, Zn,L is the nth root of JL. Dirac notation is used to describe the electronic states, |n,L>. The roots of the Bessel function are given below in terms of the n and L quantum numbers.
\[ \begin{array} & \text{L quantum number} \\ Z = \begin{pmatrix} 0 & 1 & 2 & 3 & 4 & 5 & 6 & 7 & "n" \\ 2.405 & 3.832 & 5.316 & 6.380 & 7.588 & 8.771 & 9.936 & 11.086 & 1 \\ 8.654 & 10.173 & 11.620 & 13.015 & 14.373 & 15.700 & 17.004 & 18.288 & 3 \\ 11.792 & 13.324 & 14.796 & 16.223 & 17.616 & 18.980 & 20.321 & 21.642 & 4 \\ 14.931 & 16.471 & 17.960 & 19.409 & 20.827 & 22.218 & 23.586 & 24.935 & 5 \end{pmatrix} & \text{n quantum number} \end{array} \nonumber \]
The manifold of allowed energy levels up to the LUMO is shown below and is populated with 6 π electrons. Note that the states with L > 0 are doubly degenerate.
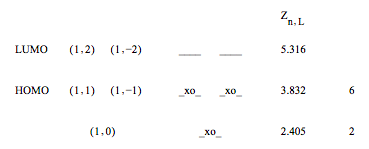
The photon wavelength required for the first electronic transition involving the π electrons is now calculated. The ring circumference is approximated as six benzene carbon-carbon bond lengths.
\[ \begin{matrix} h = 6.6260755 (10^{-34}) \text{joule sec} & c = 2.99792458 (10^8) \frac{m}{sec} & m_e = 9.1093897 (10^{-31}) kg & pm = 10^{-12} m \end{matrix} \nonumber \]
\[ \begin{matrix} C = 6(140) pm & \begin{array}{c|c} \frac{ \left( Z_{1,~1} \right)^2 h^2}{2 m_e C^2} + \frac{hc}{ \lambda} = \frac{ \left( Z_{1,~2} \right)^2 h^2}{2 m_e C^2} & _{ \text{solve, } \lambda}^{ \text{float, 3}} \rightarrow \frac{4.28e-8 kg~m^3}{joule~sec^2} = 42.8 nm \end{array} \end{matrix} \nonumber \]
Benzene has a strong electronic transition at about 200 nm.

