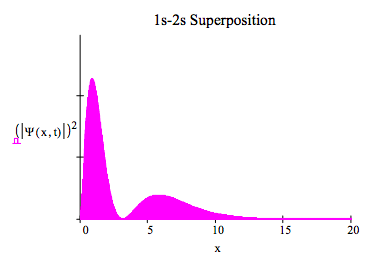
4.12: The 1s-2s Electronic Transition in the 1D Hydrogen Atom
- Page ID
- 150724
This tutorial examines the 1s-2s electronic transition in the hydrogen atom. These L = 0 states of the hydrogen atom can be written as one-dimensional, time-dependent wave functions as shown below.
The condition for an allowed electronic transition is two-fold:
- the photon exciting the transition must satisfy the Bohr frequency condition [hν = E_f - E_i ] and
- the expectation value for the position of the electron must exhibit oscillatory dipole character as a function of time.
This latter requirement provides a coupling mechanism between the oscillating electromagnetic field and the oscillating charge density of the electron. For further details see McMillin J. Chem. Ed. 55, 7 (1978).
The subsequent calculations are carried out using atomic units: \(h = 2 π\); \(m_e = 1\).
\[ \begin{matrix} \text{Initial state:} & n_i = 1 & E_i = \frac{-0.5}{n_i^2} & \text{Final state:} & n_f = 2 & E_f = \frac{-0.5}{n_f^2} \end{matrix} \nonumber \]
Time-dependent 1s and 2s electron wave functions:
\[ \begin{matrix} \Psi_1 (x,~t) = 2 x \text{exp}(-x) \text{exp}(-i E_i t) & \Psi_2 (x,~t) = \frac{1}{ \sqrt{8}} x(2-x) \text{exp} \left( - \frac{x}{2} \right) \text{exp} (-i E_f t) \end{matrix} \nonumber \]
The following graphic shows the separate 1s and 2s electron densities at \(t =0\).
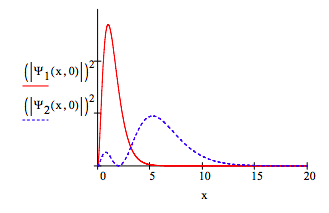
In the presence of a perturbing electromagnetic field the hydrogen atom electron is no longer in a stationary state and is represented by the following time-dependent superposition of the 1s and 2s electronic states.
\[ \Psi (x,~t) = \frac{1}{ \sqrt{2}} \left( \Psi_1 (x,~t) + \Psi_2 (x,~t) \right) \nonumber \]
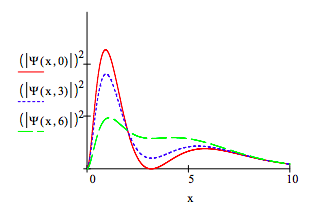
The square of the absolute magnitude for this time-dependent superposition is shown below for three time values. It clearly shows the oscillatory character of the perturbed electron density, and it appears that the oscillations have dipole character.
That the oscillations of the electron density do indeed have dipole character can also be seen by plotting the average position of the electron from the nucleus as a function of time.
\[ \begin{matrix} \text{Average Position(t)} = \int_0^{ \infty} x \left( \left| \Psi (x,~t) \right| \right)^2 dx & t = 0 .. 100 \end{matrix} \nonumber \]
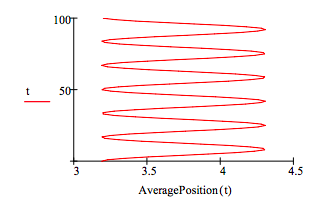
Since the expectation value for position fluctuates in time creating an oscillating dipole the transition between the \(n =1\) and \(n = 2\) states is allowed.
It is also possible to animate the time-dependent superposition. Click on Tools, Animation, Record and follow the instructions in the dialog box. Choose From: 0, To: 100, At: 10 Frames/Sec .
t = FRAME