9.9: Numerical Solutions for the Harmonic Oscillator
- Page ID
- 137727
Schrödinger's equation is integrated numerically for the first three energy states for the harmonic oscillator. The integration algorithm is taken from J. C. Hansen, J. Chem. Educ. Software, 8C2, 1996.
Set parameters:
Increments: n = 100
Integration limits: xmin = -5
xmax = 5
\[ \Delta = \frac{xmax - xmin}{n-1} \nonumber \]
Effective mass: \( \mu\) = 1
Force constant: k = 1
Calculate position vector, the potential energy matrix, and the kinetic energy matrix. Then combine them into a total energy matrix.
i = 1 .. n j = 1 .. n xi = xmin + (i - 1) \( \Delta\)
\[ V_{i,~j} = if \bigg[ i =j,~ \frac{1}{2} k (x)^2 ,~0 \bigg] \nonumber \]
\[ T_{i,~j} = if \bigg[ i=j, \frac{ \pi ^{2}}{6 \mu \Delta ^{2}}, \frac{ (-1)^{i-j}}{ (i-j)^{2} \mu \Delta^{2}} \bigg] \nonumber \]
Hamiltonian matrix: H = T + V
Find eigenvalues: E = sort(eigenvals(H))
Display three eigenvalues: m = 1 .. 3
Em =
\( \begin{array}{|r|}
\hline \\
0.5000 \\
\hline \\
1.5000 \\
\hline \\
2.5000 \\
\hline
\end{array} \)
Calculate associated eigenfunctions:
k = 1 .. 3
\[ \psi (k) = eigenvec (H, E_k) \nonumber \]
Plot the potential energy and selected eigenfunctions:
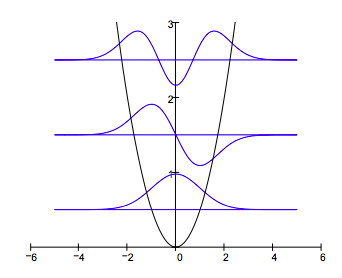
For V = axn the virial theorem requires the following relationship between the expectation values for kinetic and potential energy: <T> = 0.5n<V>. The calculations below show the virial theorem is satisfied for the harmonic oscillator for which n = 2.
\( \begin{pmatrix}
"Kinetic~Energy" & "Potential~Energy" & "Total~Energy" \\
\psi (1)^{T} T \Psi(1) & \psi (1)^{T} V \psi(1) & E_{1} \\
\psi (2)^{T} T \Psi(2) & \psi (2)^{T} V \psi(2) & E_{2} \\
\psi (3)^{T} T \Psi(3) & \psi (3)^{T} V \psi(3) & E_{3}
\end{pmatrix} = \begin{pmatrix}
"Kinetic~Energy" & "Potential~Energy" & "Total~Energy" \\
0.2500 & 0.2500 & 0.5000 \\
0.7500 & 0.7500 & 1.5000 \\
1.2500 & 1.2500 & 2.5000
\end{pmatrix} \)

