3.17: Covalent Bonding in Ammonia from Several Perspectives
- Page ID
- 152897
Chemists use a variety of models to describe the electronic structure of molecules. The oldest and most rudimentary model which is still in widespread use, and is taught at all levels of the chemistry curriculum, is the localized electron pair model proposed by G. N. Lewis in 1916. This pre-quantum approach to chemical bonding was stimulated in part by the observation that half the elements in the periodic table have an odd number of electrons, but very few molecules do.
For example ammonia, NH3, has four atoms each with an odd number of valence electrons (5,1,1,1), giving the molecule the magic number of eight valence electrons or four pairs in the Lewis formulation. In the non-geometrical representation shown below, Lewis has the nitrogen sharing a pair of electrons with each hydrogen, and keeping a lone-pair for itself.
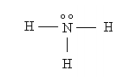
In a proper three-dimensional representation the ammonia molecule has C3v symmetry and looks like a camera tripod with the lone pair occupying the position of the camera.
However there is a serious problem with this simple model for the electronic structure of ammonia, and that is that it suggests that there are two types of valence electrons - three equivalent bonding pairs and one non-bonding pair. Unfortunately, there are three bands in ammonia's photoelectron spectrum, indicating that there are actually three types of valence electrons.
Quantum mechanical calculations based on the molecular orbital approach to chemical bonding (MO - LCAO; molecular orbitals formed as a linear combination of atomic orbitals) yield the following molecular orbitals which are in agreement with the experimental spectroscopic data.
Highest Occupied Molecular Orbital - HOMO
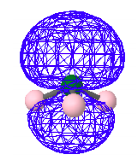
This MO is clearly non-bonding and represents the lone-pair electrons on the nitrogen.
The next three MOs, a degenerate pair and a single ground-state MO, represent the three bonding pairs of electrons. However they are not localized between the nitrogen and hydrogens as suggested by the simple Lewis structure shown above. They are indeed molecular orbitals, delocalizing the bonding electron density over the whole molecule.
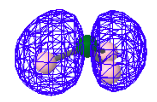
HOMO-1

HOMO-2
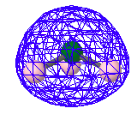
HOMO-3
Thus the delocalized canonical molecular orbitals provided by ab initio quantum mechanics do not directly support the concept of the localized electron pair model of Lewis that has proven so useful to chemists. However, the quantum mechanical superposition principle allows one to construct localized molecular orbitals (LMOs) from the canonical delocalized molecular orbitals (DMOs).
In other words, using appropriate protocols linear combinations of the canonical MOs shown above yield the LMOs shown below which are consistent with the rudimentary Lewis structure shown above.
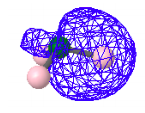
There are three localized bonding MOs like this, one for each N - H bond.
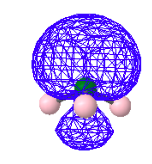
And one non-bonding MO like this.

