3.5: The H2 Covalent Bond and the Virial Theorem
- Page ID
- 151907
\[ \begin{matrix} \text{Total energy:} & E(R) = T(R) + V(R) \\ \text{Virial theorem:} & 2 T(R) + V(R) = -R \frac{d}{dR} E(R) \\ \text{Morse parameters:} & D_e = 0.761 aJ & \beta = 0.0193 pm^{-1} & R_e = 74.1 pm \end{matrix} \nonumber \]
These parameters are taken from Fig. 5.6 on page 165 of McQuarrie and Simon's Physical Chemistry: A Molecular Approach.
\[ \begin{matrix} \text{Morse Function:} & E(R) = D_e \left[ 1 - \text{exp} \left[ - \beta \left( R - R_e \right) \right] \right]^2 - D_e \end{matrix} \nonumber \]
Using equation (1) to eliminate V(R) in equation (2) yields an equation for kinetic energy as a function of the internuclear separation.
\[ \begin{matrix} \text{Kinetic energy:} & T(R) = -E(R) - R \frac{d}{dR} E(R) \end{matrix} \nonumber \]
Using equation (1) to eliminate T(R) in equation (2) yields an equation for potential energy as a function of the internuclear separation.
\[ \begin{matrix} \text{Potential energy:} & T(R) = 2E(R) + R \frac{d}{dR} E(R) \end{matrix} \nonumber \]
John C. Slater [J.Chem. Phys. 1933, 1, 687-691] used the virial theorem to analyze the chemical bond and was among the first to note the importance of kinetic energy in covalent bond formation. He wrote,
...this theorem gives a means of finding kinetic and potential energy separately for all configurations of the nuclei, as soon as the total energy is known, from experiment or theory.
These important equations (4 and 5) determine the mean kinetic and potential energies as functions of R, one might almost say experimentally, directly from the curves of E as a function of R which can be found from band spectra. The theory is so simple and direct that one can accept the results without question....
John Winn [J. Chem. Phys. 1981, 74, 608-611] offered the following interpretation of the energy profile shown below.
As the atoms approach, the potential energy rises (electrons are moving away from nuclei) and the kinetic energy falls (as delocalization begins). In the vicinity of R/Re = 2, this trend reverses. The kinetic energy increases as the wave function is localized ..., but the potential energy falls as the charge is now brought nearer both nuclei. Only at R/Re < 0.5 does nuclear repulsion cause the potential energy to increase and contribute to the total repulsion energy.
\[ \begin{matrix} \text{Plot range:} & R = 30 pm,~35 pm .. 400 pm \end{matrix} \nonumber \]
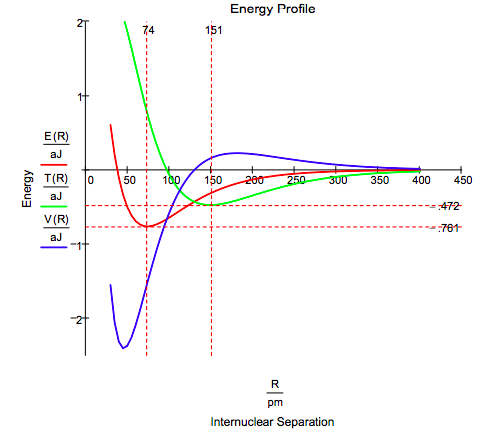
The following table provides quantitative support for Winn's analysis.
\[ \begin{pmatrix} o & \text{Step 1} & \text{Step 2} & \text{Overall} \\ \frac{ \Delta T}{aJ} & -0.472 & 1.233 & 0.761 \\ \frac{ \Delta V}{aJ} & 0.166 & -1.688 & -1.522 \\ \frac{ \Delta E}{aJ} & -0.306 & -0.455 & -0.761 \\ \frac{R}{pm} & 151 & 74.1 & 74.1 \\ \text{Type} & \text{Molecular} & \text{Atomic} & \text{Molecular + Atomic} \end{pmatrix} \nonumber \]
This type of analysis is valid for any diatomic molecule for which Morse paramters are available.
\[ \begin{matrix} \text{Conversion factors:} & pm = 10^{-12} m & aJ = 10^{-18} joule \end{matrix} \nonumber \]

