2.44: The Crucial Role of Kinetic Energy in Interpreting Ionization Energies
- Page ID
- 158375
This Journal has recently published a series of four articles by Gillespie, Spencer, and Moog under the banner of “Demystifying Introductory Chemistry”, an effort supported by the Task Force on General Chemistry (1). In their opening remarks they make the following statement (1a):
In our opinion we make chemistry seem more abstract, more mysterious, and more esoteric than necessary. Chemistry is certainly a complicated subject, but shrouding it in esoteric jargon and impenetrable theory makes it seem much more difficult than it really is.
We accept many of the excellent recommendations the authors make for improving the general chemistry sequence, but we have serious reservations about one of their arguments. In their attempt to draw back the quantum mechanical veil shrouding introductory chemistry they offer an incorrect interpretation of the trend of ionization energies for the first two elements of the periodic table and carry this form of reasoning forward to Li and Be. We quote one of their paragraphs (1a) in its entirety and then proceed to our objections and a quantum mechanical analysis.
The first ionization energy of helium (2.37 MJ mol-1) is nearly twice that of hydrogen (1.31 MJ mol-1); thus, these ionization energies are consistent with the two electrons in helium being at about the same distance from the nucleus as the single electron in hydrogen. These two electrons occupy a spherical region around the nucleus— the first (n = 1) shell. The ionization energy of helium is slightly less than twice the ionization energy of hydrogen because of the repulsion between the two electrons in helium.
We find two problems with this paragraph. The first is the statement that the two electrons in helium are about the same distance from the nucleus as the hydrogen electron. We do not believe the experimental ionization energies themselves provide support for this assertion. Furthermore, we do not believe there is other reliable experimental or theoretical evidence that supports the assertion. The second is the fact that the authors have used a classical explanation that is based solely on potential energy (Coulomb’s law, potential energy = -Ze2/r): the electrons are about the same distance from the nucleus, the nuclear charge increases by a factor of two, so the attraction to the nucleus increases by a factor of two, but the ionization energy increases by a factor of only 1.81 because of electron–electron repulsion. What is missing in the above potential energy argument is the fact that kinetic energy is an important factor in the quantum world of atoms and molecules, and cannot be ignored. More than three decades ago Ruedenberg (2) demonstrated the crucial importance of electron kinetic energy in understanding the physical nature of the chemical bond. Unfortunately, in spite of discussion of this profoundly important analysis in the pedagogical literature (3–5) and review journals (2b, 6), Ruedenberg’s work has been largely ignored by the undergraduate chemistry community. We hope that the quantum mechanical treatment that we present will help to underline the role of kinetic energy in understanding atomic structure and stability, and in particular its importance in understanding the ionization energy ratio of 1.81 mentioned above.
The hydrogen atom problem can be solved exactly, but the two-electron helium atom cannot. However, it can be solved to an arbitrary degree of accuracy by approximate methods. So the first issue is what level of theory should be employed in order to achieve an understanding of the problem under study. In our analysis, hydrogenic 1s orbitals
\[ \Psi = \sqrt{ \frac{ \alpha^3}{ \pi}} \text{exp} ( - \alpha r) \nonumber \]
will be used with the variational principle to obtain the electron orbital energies for the hydrogen and helium atoms. The orbital energies will be used because Koopmans’ theorem (7) states that the ionization energy can be approximated as the negative of the orbital energy. This is correct for the hydrogen atom, but approximate for the helium atom. However, as we shall see, the error introduced by this approximation is relatively small. We show that this simple quantum mechanical analysis gives satisfactory agreement with experimental results and provides a basis from which to reach an interpretation of the relative values of the ionization energies of hydrogen and helium.
Use of the trial wave function chosen above with the variational theorem yields the results given in the tables. Table 1 shows the expressions for the total electron energy, the electron orbital energies, and the optimum values of obtained when the variational principle is applied to the total energy. Table 2 gives the results of the variational calculation for the electronic orbital energy for hydrogen and helium.
Table 1. Total Electron and Electron Orbital Energy for H and He
\[ \begin{array}{|c c c c|} \hline \hline \text{Element} & \text{Total Electron Energy} & \text{Electron Orbital Energy} & \text{Optimum } \alpha \\ \hline H~ (Z = 1) & E_H = \frac{ \alpha^2}{2} - Z_{ \alpha} & \varepsilon = \frac{ \alpha^2}{2} - Z_{ \alpha} & \alpha = 1 \\ He ~(Z = 2) & E_{He} = \alpha^2 - 2Z_{ \alpha} + \frac{5 \alpha}{8} & \varepsilon_{He} = \frac{ \alpha^2}{2} - Z_{ \alpha} + \frac{5 \alpha}{8} & \alpha = 1.6875 \\ \hline \end{array} \nonumber \]
The orbital energies are important because we will make use of Koopmans’ theorem in our analysis. Appendix A provides a brief discussion of the use of Koopmans’ theorem in interpreting the ionization energy of the helium atom. Atomic units have been used because they are properly scaled for atomic calculations. The conversion factor to SI units is 1 hartree = 2.6255 MJ mol-1 (8).
We now discuss the entries in these tables. For the hydrogen atom there are two contributions to the electronic energy, EH: kinetic energy (α2/2) and the electrostatic interaction with the nucleus (-Zα). Because there is only one electron in the hydrogen atom, this is also the expression for the electron orbital energy. Minimization of EH with respect to α yields α = 1, EH = -0.5 hartree, and εH = -0.5 hartree. Applying Koopmans’ theorem (IE = -εH) yields an ionization energy of 0.5 hartree, which is in agreement with experiment. The evaluation of the variational integrals that appear in the hydrogen and helium calculations outlined in this paper can be found in papers previously published in this Journal (9, 10).
For the helium atom with two electrons there are five contributions to the total electronic energy, EHe: the kinetic energy of each electron (α2/2), the electrostatic interaction of each electron with the nucleus (-Zα), and the electrostatic interaction of the electrons with each other (5α/8). The electron orbital energy for the helium atom, εHe, is simply those energy contributions that an individual electron experiences: kinetic energy (α2/2), electrostatic interaction with the nucleus (-Zα), and the electrostatic interaction with the other electron (5α/8). Minimization of EHe with respect to α yields α = 1.6875, EHe = -2.848 hartree, and εHe = -0.8965 hartree. Appendix B provides a graphical representation of the variational procedure. Thus, the predicted ionization energy using Koopmans’ theorem is 0.8965 hartree. The experimental ionization energy is 0.9037 hartree, so this result is not exact; but it is reasonably close, given the simplicity of the wave function chosen.
The first thing to note is that this analysis yields a ratio of ionization energies (IE ≈ -ε) of 1.79, as compared with the experimental value of 1.81. This gives us some confidence that we have chosen a reliable level of theory to deal with the question at hand. Note also that this quantum mechanical treatment brings into serious question the assumption (1a) that the electrons are about the same distance from the nucleus in the hydrogen atom and the helium atom. The last row of the second table shows that the ratio of , the quantum mechanically calculated average radial distance from the nucleus, is 0.59 which is considerably different from 1.0.
Continuing with the results given in the second table, we note that in going from H to He the orbital potential energy more than doubles (2.32). The orbital potential energy in He is the sum of the electron–nuclear attraction (-2α = -3.375 hartree) and the electron–electron repulsion (5α/8 = 1.055 hartree) terms. Thus we see that the electron–nuclear attraction more than triples, but the total potential energy increases by a factor of only 2.32 because of electron–electron repulsion. Coming back to the original issue, that the ionization energy increases by a factor of only 1.81 from H to He, we can now see that the explanation must lie in the kinetic energy term. Table 2 shows that the orbital kinetic energy almost triples (2.85) in going from H to He. Thus, the reason for the less than doubling of the ionization energy cannot be found by considering only potential energy, which alone predicts more than a doubling of the ionization energy. The explanation for the less than doubling of the ionization energy actually lies in the large increase in electron kinetic energy as the electrons are drawn closer to the nucleus by the increase in nuclear charge from H to He.
Table 2. Variational Results for Hydrogen and Helium
\[ \begin{array}{|c c c c|} \hline \hline \text{Parameter} & \text{Hydrogen} & \text{Helium} & \text{Ratio, He/H} \\ \hline \text{Electron orbital energy} & \varepsilon_H = \alpha^2/2 - \alpha & \varepsilon_{He} = \frac{ \alpha^2}{2} - 2 \alpha + \frac{5 \alpha}{8} \\ \text{Optimum, } \alpha & 1.0 & 1.6875 & 1.6875 \\ \text{Exp. ionization energy} & 0.5 & 0.9037 & 1.81 \\ \varepsilon ~ \text{(orbital energy)} & -0.5 & -0.8965 & 1.79 \\ \text{Kinetic energy} & 0.5 & 1.424 & 2.85 \\ \text{Potential energy} & -1.0 & -3.375 + 1.055 = -2.32 & 2.32 \\ \langle R \rangle & 1.5 & 0.89 & 0.59 \end{array} \nonumber \]
Earlier we referenced Ruedenberg’s analysis of the physical nature of the chemical bond. What he said about molecular bonding also pertains to atomic electronic structure (2c):
Finally, it should be emphasized that the phenomenon of the eigenstate is intimately related to the fact that molecules are subject to the laws of quantum mechanics; there are no ground states in classical mechanics or electrostatics [emphasis added]. Consequently, a physical picture seeking to describe chemical bonding must necessarily incorporate features which distinguish quantum mechanics from classical mechanics and electrostatics…It may be added that the existence of a ground state is intrinsically connected with the fact that the variation integral contains both kinetic and potential energy…Omission of one or the other from consideration cannot, therefore, lead to a full interpretation of binding.
We have seen that one needs to use a quantum chemical treatment to understand the ratio of ionization energies for H and He. We wish to point out that interpreting the ionization of any atom or molecule requires quantum chemical tools and a consideration of both kinetic and potential energy.
While the quantum mechanical arguments outlined here may be used with undergraduate physical chemistry students, they are obviously too advanced for introductory students. However, we also should not use incorrect classical models. This leaves us with the important task of deciding what we can say to introductory students about the details of the periodicity of physical properties, such as ionization energies, that is both correct and understandable.
Literature Cited
- (a) Gillespie, R. J.; Spencer, J. N.; Moog, R. S. J. Chem. Educ. 1996, 73, 617. (b) Gillespie, R. J.; Spencer, J. N.; Moog, R. S. Ibid., 622. (c) Spencer, J. N.; Moog, R. S.; Gillespie, R. J. Ibid., 627. (d) Spencer, J. N.; Moog, R. S.; Gillespie, R. J. Ibid., 631.
- (a) Ruedenberg, K. Rev. Mod. Phys. 1962, 34, 326. (b) Feinberg, M. J.; Ruedenberg, K.; Mehler, E. L. Adv. Quantum Chem. 1970, 5, 27. (c) Ruedenberg, K. In Localization and Delocalization in Quantum Chemistry, Vol. I; O. Chalvet et al., Eds.; D. Reidel: Dordrecht, 1975; pp 223–245.
- Baird, N. C. J. Chem. Educ. 1986, 63, 660.
- DeKock, R. L. J. Chem. Educ. 1987, 64, 934.
- Harcourt, R. D. Am. J. Phys. 1988, 56, 660.
- Kutzelnigg, W. Angew. Chem. Int. Ed. Eng. 1973, 12, 546.
- Koopmans, T. A. Physica 1933, 1, 104. Lowe, J. P. Quantum Chemistry, 2nd ed.; Academic: New York, 1993; pp 361–363.
- International Union of Pure and Applied Chemistry. Quantities, Units, and Symbols in Physical Chemistry, 2nd ed.; Blackwell Scientific: Oxford, 1993. Almost any physical chemistry or quantum chemistry textbook will also make reference to “atomic units”, for which the energy unit is called the “hartree.”
- Snow, R. L.; Bills, J. L. J. Chem. Educ. 1975, 52, 506.
- Lee, S.-Y. J. Chem. Educ. 1983, 60, 935.
- Linnett, J. W. Wave Mechanics and Valency; Methuen: London, 1960; pp 1–2.
- Atkins, P. W. Physical Chemistry, 5th ed.; Freeman: New York, 1994; p 371.
Appendix
A. Employing Koopmans’ theorem at the Hartree-Fock level to interpret the helium atom ionization energy breaks the ionization process into two steps: (1) frozen ionization (constant α) followed by (2) relaxation to the He+ ground state:
\[ He ( \alpha = 1.6875) \rightarrow He^{+} ( \alpha = 1.6875);~ \Delta E = - \varepsilon = 0.89648~ \text{hartree} \nonumber \]
\[ He^+ ( \alpha=1.6875) \rightarrow He^+ ( \alpha = 2);~ \Delta E = -0.0488 \text{ hartree} \nonumber \]
One reason Koopmans’ theorem is successful in approximating the ionization in this way is that the energy change accompanying relaxation to the true ground state of the helium ion is small. Another reason is that Hartree–Fock calculations ignore electron correlation. With the wave function used in this study the correlation energy, EC , is
\[ E_C = IT_{exp} - \left( E_{He+} - E_{He} \right) = 0.9037 - (-2.0000 + 2.8478) = 0.0560 \text{ hartree} \nonumber \]
Thus, the correlation (0.0560 hartree) and relaxation ({0.0488 hartree) energy nearly cancel. This cancellation of terms accounts for the better-than-expected agreement between theory and experiment, given the simplicity of the wave function chosen. B. The variational expression for the helium atom energy using the wave function chosen in this study is
\[ E( \alpha ) = \alpha^2 - 4 \alpha + \frac{5 \alpha}{8} \nonumber \]
Using the fact that the average radial distance of the electrons from the nucleus is inversely proportional to the scale factor α, = 1.5/α, R becomes the variational parameter and the variation integral becomes
\[ E(R) = \frac{9}{4R^2} - \frac{6}{R} + \frac{15}{16R} = T(R) + V_{en} (R) + V_{ee} (R) \nonumber \]
The figure below provides a graphical representation of the variation method (minimization of E with respect to R) and shows the behavior of the kinetic energy (T), electron–nuclear potential energy (Ven), electron–electron potential energy (Vee), and total electronic energy (E) as a function of , the average radial distance of the helium atom electrons from the nucleus.
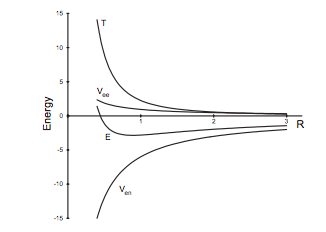
Contributions to the electronic energy of the He atom
Note the relative insignificance of Vee compared to T and Ven. In other words, T and Ven are the dominant terms contributing to the ground state of the helium atom. This figure also clearly illustrates that the existence of a ground state (2) depends on the kinetic energy term. Atomic and molecular stability, therefore, can only be understood in quantum mechanical terms, and the foundation of all quantum mechanics is de Broglie’s hypothesis that matter has wavelike properties, λ = h/mv. For one-dimensional problems it is easy to show the relationship between Schrödinger’s equation and de Broglie’s wave equation (11, 12).

