4.11: Quantum Beats
- Page ID
- 150723
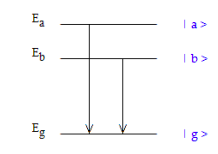
Quantum beats are the oscillatory behavior in the intensity of radiation emitted by atomic or molecular systems that are in a superposition of excited states created by off‐resonance excitation. The quantum mechanical analysis of quantum beats is essentially the same as it is for the double‐slit experiment. In the double‐slit experiment there are two paths to each position on the detector and the probability amplitudes for these paths interfere. As shown in the figure below, in this example the ground‐state can be reached from two excited states having different energies. The probability amplitudes for these paths to the ground state (| a > ‐‐‐> | g > and | b > ‐‐‐> | g >) also interfere constructively and destructively causing oscillations in the intensity of the emitted radiation.
The superposition of transitions to the ground state in the time domain is as follows,
\[ \langle g | t \rangle \langle t | a \rangle + \langle g | t \rangle \langle t | b \rangle \nonumber \]
where, for example, the Dirac brackets in atomic units are of the form
\[ \langle t | a \rangle = \langle a | t \rangle* = \text{exp}(-i E_a t) \nonumber \]
Using relations of the type given in equation (2) we can write equation (1) as
\[ \text{exp}(iE_g t) \text{exp}(-iE_a t) + \text{exp}(iE_g t) \text{exp}(-i E_b t) \nonumber \]
This equation can be simplified by using the ground state as a reference by setting Eg = 0.
\[ \text{exp}(-iE_a t) + \text{exp}(-iE_b t) \nonumber \]
The excited states decay exponentially, but not necessarily with the same decay constant. The fraction of the atoms or molecules not decayed at time t is given by first‐order kinetics.
\[ \frac{A_t}{A_0} = \text{exp} (-k_a t) \nonumber \]
Thus, each term in equation (4) is weighted by the probability that state exists at time t.
\[ \text{exp}(-i E_a t) \text{exp}(-k_a t) + \text{exp} (-iE_b t) \text{exp}(-k_b t) \nonumber \]
The time‐dependence of the intensity of the emitted radiation is proportional to the square of the absolute magnitude of equation (6)
\[ \left| \text{exp} (-iE_a t) \text{exp} (-k_a t) + \text{exp}(-i E_b t) \text{exp} (-k_bt) \right|^2 \nonumber \]
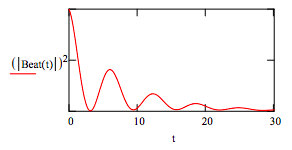
The time‐dependence as calculated below for arbitrary values for the parameters clearly shows the oscillatory character of the intensity of the emitted radiation.
\[ \begin{matrix} E_a = 1 & k_a = .1 & E_b = 2 & k_b = .05 \end{matrix} \nonumber \]
\[ \text{Beat(t)} = \text{exp} (-i E_b t) \text{exp} (-k_b t) + \text{exp}(-i E_a t) \text{exp}(-k_a t) \nonumber \]