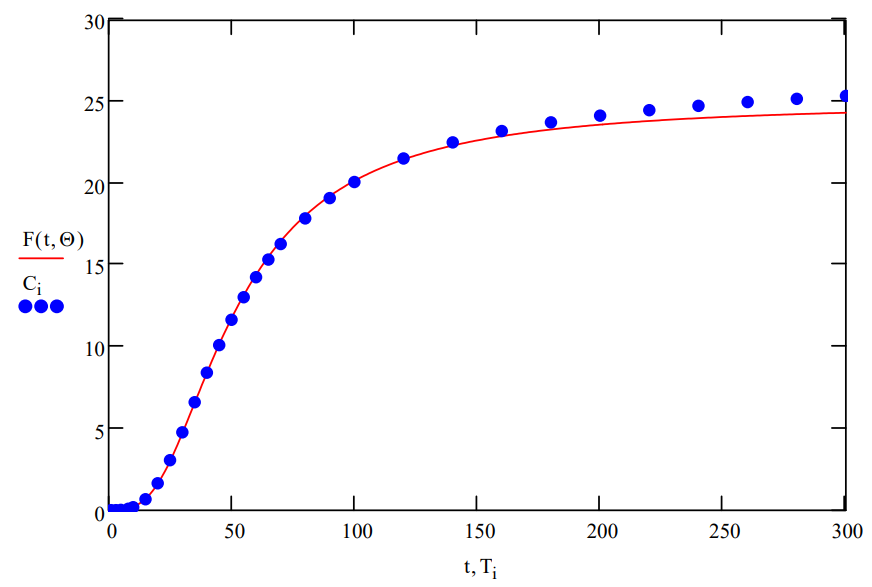
1.91: Fitting Debye's Heat Capacity Equation to Experimental Data for Silver
- Page ID
- 158510
\[
\mathrm{n} :=30 \qquad \mathrm{i} :=1 \ldots \mathrm{n}
\nonumber \]
| \(\mathrm{T}_{\mathrm{i}} :=\) | \(\mathrm{C}_{\mathrm{i}} :=\) |
| 1 | 0.000818 |
| 3 | 0.0065 |
| 5 | 0.0243 |
| 8 | 0.0927 |
| 10 | 0.183 |
| 15 | 0.670 |
| 20 | 1.647 |
| 25 | 3.066 |
| 30 | 4.774 |
| 35 | 6.612 |
| 40 | 8.419 |
| 45 | 10.11 |
| 50 | 11.66 |
| 55 | 13.04 |
| 60 | 14.27 |
| 65 | 15.35 |
| 70 | 16.30 |
| 80 | 17.87 |
| 90 | 19.11 |
| 100 | 20.10 |
| 120 | 21.54 |
| 140 | 22.52 |
| 160 | 23.22 |
| 180 | 23.75 |
| 200 | 24.16 |
| 220 | 24.49 |
| 240 | 24.76 |
| 260 | 24.99 |
| 280 | 25.19 |
| 300 | 25.37 |
The heat capacity data were taken from the Handbook of Physics and Chemistry ‐ 72nd Edition, page 5‐71. The data are presented in units of Joules/mole/K.
Gas law constant:
\[
\mathrm{R} :=8.31451
\nonumber \]
Define Debye function for heat capacity:
\[
\mathrm{F}(\mathrm{T}, \Theta) :=9 \cdot \mathrm{R} \cdot\left(\frac{\mathrm{T}}{\Theta}\right)^{3} \cdot \int_{0}^{\frac{\Theta}{\mathrm{T}}} \frac{x^{4} \cdot \exp (\mathrm{x})}{(\exp (\mathrm{x})-1)^{2}} \mathrm{dx} \quad \text{where} x = \frac{hv}{kT}
\nonumber \]
Form the sum of the squares of the deviations:
\[
\operatorname{SSD}(\Theta) :=\sum_{\mathrm{i}}\left(\mathrm{C}_{\mathrm{i}}-\mathrm{F}\left(\mathrm{T}_{\mathrm{i}}, \Theta\right)\right)^{2}
\nonumber \]
Minimize the sum of the squares of the deviations:
\[
\Theta :=200
\nonumber \]
Given
\[
\operatorname{SSD}(\Theta)=0 \qquad \Theta :=\operatorname{Minerr}(\Theta)
\nonumber \]
Debye Temperature for best fit:
\[
\Theta=210.986
\nonumber \]
Mean squared error:
\[
\frac{\operatorname{SSD}(\Theta)}{(n-2)}=0.16
\nonumber \]
Plot data and fit:
\[
t :=1 \ldots 300
\nonumber \]