2.1: How do I choose a reference standard for my Q-NMR analysis?
- Page ID
- 77769
With NMR, we need only to have available any pure standard compound (which can be structurally unrelated to our analyte) that contains the nucleus of interest and has a resonance that does not overlap those of our analyte. The analyte concentration can then be determined relative to this standard compound. The requirement for lack of overlap means that most standards have simple NMR spectra, often producing only singlet resonances. Additional requirements for standards to be used for quantitative analysis are that they:
- are chemically inert
- have low volatility
- have similar solubility characteristics as the analyte
- have reasonable T1 relaxation times
The structures of several common NMR chemical shift and quantitation standards are shown in the figure below.
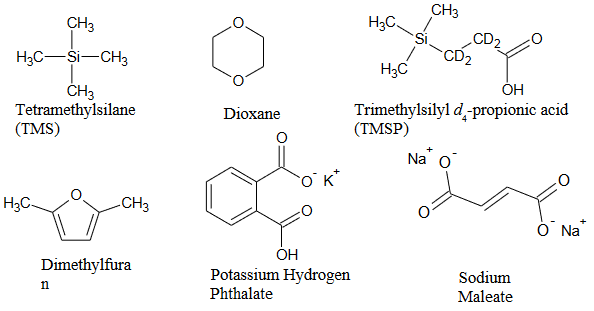
TMS and dioxane are chemical shift reference compounds commonly used in organic solvents. However they do not make good quantitation standards because they suffer from high volatility. Therefore it is difficult to prepare a standard solution for which the concentration is known with high accuracy. TMSP is a water soluble chemical shift reference. While it has improved performance as a quantitation standard compared with TMS or dioxane, it has been shown to absorb to glass so stock solutions may have stability problems.1 In addition to the criteria listed above, it is helpful for quantitation purposes if the compound selected as the standard also has the properties of a primary analytical standard, for example potassium hydrogen phthalate (KHP), which is available in pure form, is a crystalline solid at room temperature and can be dried to remove waters of hydration.


