6.3: Diabatic and Adiabatic States
- Page ID
- 107246
Although the Born–Oppenheimer surfaces are the most straightforward and commonly calculated, they may not be the most chemically meaningful states. As an example consider the potential energy curves for the diatomic \(\ce{NaCl}\). The chemically distinct potential energy surfaces one is likely to discuss have distinct atomic or ionic character at large separation between the atoms. These “diabatic” curves focus on physical effects, but are not eigenstates. In the figure, the ionic state \(| a \rangle\) is influenced by the Coulomb attraction between ions that draws them together, leading to a stable configuration at \(R_{eq}\) once these attractive terms are balanced by nuclear repulsive forces. However, the neutral atoms (\(\ce{Na^{0}}\) and \(\ce{Cl^{0}}\)) have a potential energy surface \(| b \rangle\) which is dominated by repulsive interactions. The adiabatic potentials from the BO Hamiltonian will reflect significant coupling between the diabatic electronic states. BO states of the same symmetry will exhibit an avoided crossing where the electronic energy between corresponding diabatic states is equal. As expected from our earlier discussion, the splitting at the crossing for this one-dimensional system would be \(2V_{ab}\), twice the coupling between diabatic states.
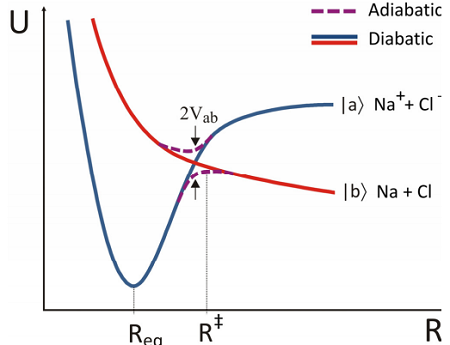
The adiabatic potential energy surfaces are important in interpreting the reaction dynamics, as can be illustrated with the reaction between \(\ce{Na}\) and \(\ce{Cl}\) atoms. If the neutral atoms are prepared on the ground state at large separation and slowly brought together, the atoms are weakly repelled until the separation reaches the transition state \(R^‡\). Here we cross into the regime where the ionic configuration has lower energy. As a result of the nonadiabatic couplings, we expect that an electron will transfer from \(\ce{Na^{0}}\) to \(\ce{Cl^{0}}\), and the ions will then feel an attractive force leading to an ionic bond with separation \(R_{eq}\).
Diabatic states can be defined in an endless number of ways, but only one adiabatic surface exists. In that respect, the term “nonadiabatic” is also used to refer to all possible diabatic surfaces. However, diabatic states are generally chosen so that the nonadiabatic electronic couplings in Equation 6.2.14 and 6.2.15 are zero. This can be accomplished by making the electronic wavefunction independent of \(R\).
As seen above, for coupled states with the same symmetry the couplings repel the adiabatic states and we get an avoided crossing. However, it is still possible for two adiabatic states to cross. Mathematically this requires that the energies of the adiabatic states be degenerate (\(E _ {\alpha} = E _ {\beta}\)) and that the coupling at that configuration be zero (\(V _ {\alpha \beta} = V _ {\beta \alpha} = 0\)). This isn’t possible for a one-dimensional problem, such as the \(\ce{NaCl}\) example above, unless symmetry dictates that the nonadiabatic coupling vanishes. To accomplish this for a Hermitian coupling operator you need two independent nuclear coordinates, which enable you to independently tune the adiabatic splitting and coupling. This leads to a single point in the two-dimensional space at which degeneracy exists, which is known as a conical intersection (an important topic that is not discussed further here).


