17.11: Half-reactions and Half-cells
- Page ID
- 152728
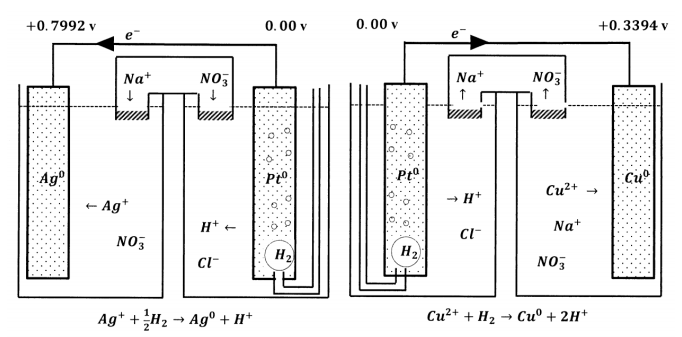
Let us consider some standard electrochemical cells we could construct using the S.H.E. Two possibilities are electrochemical cells in which the second electrode is the standard silver–silver ion electrode or the standard copper–cupric ion electrode. The diagrams in Figure 7 summarize the half-reactions and the electrical potentials that we find when we construct these cells.
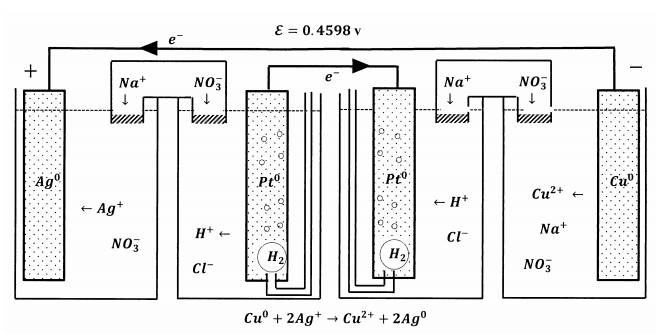
We can also connect these cells so that the two S.H.E. are joined by one wire, while a second wire joints the silver and copper electrodes. This configuration is sketched in Figure 8. Whatever happens at one S.H.E. happens in the exact reverse at the other S.H.E. The net effect is essentially the same as connecting the silver– silver ion half-cell to the copper–cupric ion half-cell by a single salt bridge. If we did not already know what reaction occurs, we could figure it out from the information we have about how each of these two cells performs when it operates against the S.H.E.