6.5: Equilibria in Chemical Reactions
- Page ID
- 151701
Equilibria involving chemical reactions share important characteristics with phase and distribution equilibria. In Chapter 5, we develop the equilibrium constant expression from ideas about reaction rates. For the present comparison, let us consider the equilibrium between the gases nitrogen dioxide, \(NO_2\), and dinitrogen tetroxide, \(N_2O_4\):
\[\ce{N_2O_4\ (g) <=> 2NO_2 (g)} \nonumber \]
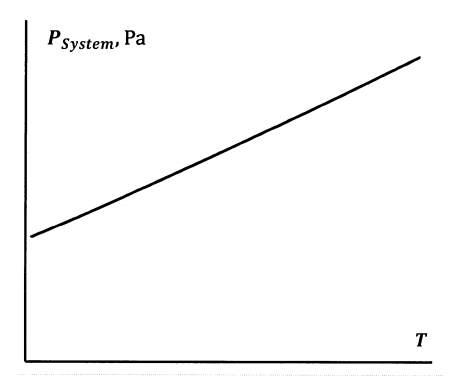
Suppose that we trap a quantity of pure \(N_2O_4\) in a cylinder closed with a piston. If we fix the temperature and volume of this system, the dissociation reaction occurs until equilibrium is achieved at some system pressure. For present purposes, let us assume that both \(N_2O_4\) and \(NO_2\) behave as ideal gases. The equilibrium system pressure will be equal to the sum of the partial pressures: \(P=P_{N_2O_4}+P_{NO_2}\). If we now do a series of experiments, in which we hold the volume constant while allowing the temperature to change, we find a continuous series of pressure–temperature combinations at which the system is at equilibrium. This curve is sketched in Figure 5. It is much like the curve describing the dependence of the water–water-vapor equilibrium on pressure and temperature.
If we hold the temperature constant and allow the volume to vary, we can change the force on the piston to keep the total pressure constant at a new value, \(P^*_{total}\). The position of the chemical equilibrium will change. At the new equilibrium position, the new \(N_2O_4\) and \(NO_2\) partial pressures will satisfy the total pressure relationship. When we repeat this experiment, we find that, whatever the total pressure, the equilibrium partial pressures are related to one another as sketched in Figure 6.
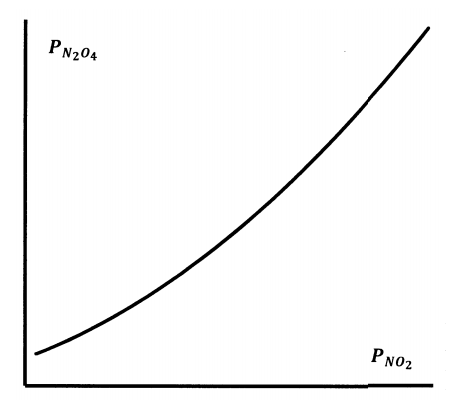
We find that the experimental data fit the equation
\[K_P= \dfrac{P^2_{NO_2}}{P_{N_2O_4}}, \nonumber \]
where \(K_P\) is the equilibrium constant for the reaction. (Pressure is a measure of gas concentration. Later, we see that the equilibrium constant can be expressed more rigorously as a ratio of fugacities—or activities.)


