6.3: Equilibrium and Reversibility - Phase Equilibria
- Page ID
- 151699
To review the general characteristics of phase equilibria, let us consider a familiar system. Suppose that we have a transparent but very strong cylinder, sealed with a frictionless piston, within which we have trapped a quantity of pure liquid water at some high pressure. We can fix the pressure of the liquid water at any value we choose by applying an appropriate force to the piston. Suppose that we hold the temperature constant and force the volume to increase by withdrawing the piston in very small increments. Because pure water is not compressed easily, we find initially that the pressure of the water decreases and does so in very large increments.
However, after some small increase in the volume, we find that imposing a further volume increase changes the system’s behavior abruptly. The system undergoes a profound change. What was formerly pure liquid becomes a mixture of liquid and gas. As we impose still further volume increases, the pressure of the system remains constant, additional liquid passes from the liquid to the gas phase, and we find that we must supply substantial amounts of heat in order to keep the temperature of the system constant. If we continue to force volume increases in this manner, vaporization continues until all of the liquid evaporates.
If we impose a decrease in the volume of the two-phase system, we see the process reverse. The pressure of the system remains constant, some of the gas condenses to liquid, and the system gives up heat to the surroundings. For any given temperature, these conversions are precisely balanced at some particular pressure, and these conditions characterize a state of liquid–vapor equilibrium. At any given pressure, the equilibrium temperature is called the boiling point of the liquid. The equilibrium pressure and temperature completely specify the state of the system, except for the exact amounts of liquid and gaseous water present.
If we begin with this system in a state of liquid–vapor equilibrium, we can increase the amount of vapor by imposing a small volume increase. Conversely, we can decrease the amount of vapor by imposing a very small volume decrease. At the equilibrium temperature and pressure, changing the imposed volume by an arbitrarily small amount (from \(V\) to \(V\pm dV\)) is sufficient to reverse the direction of the change that occurs in the system. We call any process whose direction can be reversed by an arbitrarily small change in a thermodynamic state function a reversible process. Evidently, there is a close connection between reversible processes and equilibrium states. If a process is to occur reversibly, the system must pass continuously from one equilibrium state to another.
In this description, the reversible, constant-temperature vaporization of water is driven by arbitrarily small volume changes. The system responds to these imposed volume changes so as to maintain a constant equilibrium vapor pressure at the specified temperature. We say that the reversible process “takes place at constant pressure and temperature.” We can also describe this process as being driven by arbitrarily small changes in the applied pressure: If the applied pressure exceeds the equilibrium vapor pressure by an arbitrarily small increment, \(dP>0\), condensation occurs; if the applied pressure is less than the equilibrium vapor pressure by an arbitrarily small increment, \(dP<0\), vaporization occurs. To describe this tersely, we introduce a figure of speech and say that the reversible process occurs “while the system pressure and the applied pressure are equal.” Literally, of course, there can be no change when these pressures are equal.
To cause water to vaporize at a constant temperature and pressure, we must add heat energy to the system. This heat is called the latent heat of vaporization or the enthalpy of vaporization, and it must be supplied from some entity in the surroundings. When water vapor condenses, this latent heat must be removed from the system and taken up by the surroundings. (The enthalpy change for vaporizing one mole of a substance is usually denoted \({\Delta }_{vap}H\). It varies with temperature and pressure. Tables usually give experimental values of the equilibrium boiling temperature at a pressure of 1 bar or 1 atm; then they give the enthalpy of vaporization at this temperature and pressure. We discuss the enthalpy function in Chapter 8.)
Four conditions are sufficient to exactly specify either the initial or the final state: the number of moles of liquid, the number of moles of gas, the pressure, and the temperature. The change is a conversion of some liquid to gas, or vice versa. We can represent this change as a transition from an initial state to a final state where \(n^o_{liquid}\) and \(n^o_{gas}\) are the initial numbers of moles of liquid and gas, respectively, and \(\delta n\) is the incremental number of moles vaporized: \[\left(P,\ T,n^o_{liquid},n^o_{gas}\right)\to \left(P,\ T,n^o_{liquid}-\delta n,n^o_{gas}+\delta n\right) \nonumber \]
The initial pressure and temperature are the same as the final pressure and temperature. Effecting this change requires that a quantity of heat, \(\left({\Delta }_{vap}H\right)\delta n\), be added to the system, without changing the temperature of the system.
This introduces another requirement that a reversible process must satisfy. If the reversibly vaporizing water is to take up an arbitrarily small amount of heat, the system must be in contact with surroundings that are hotter than the system. The temperature difference between the system and its surroundings must be arbitrarily small, because we can describe exactly the same process as being driven by contacting the system, at temperature \(T\), with surroundings at temperature \(\hat{T}+\delta \hat{T}\). If we keep the applied pressure constant at the temperature-\(T\) equilibrium vapor pressure, the system volume increases. We can reverse the direction of change by changing the temperature of the surroundings from \(\hat{T}+\delta \hat{T}\) to \(\hat{T}-\delta \hat{T}\). If the process is to satisfy our criterion for reversibility, the difference between these two temperatures must be arbitrarily small. To describe this requirement tersely, we again introduce a figure of speech and say that the reversible process occurs “while the system temperature and the surroundings temperature are equal.”
If we repeat the water-in-cylinder experiment with the temperature held constant at a slightly different value, we get similar results. There is again a pressure at which the process of converting liquid to vapor is at equilibrium. At this temperature and pressure, both liquid and gaseous water can be present in the system, and, so long as no heat is added or removed from the system, the amount of each remains constant. When we hold the pressure of the system constant at the equilibrium value and supply a quantity of heat to the system, a quantity of liquid is again converted to gaseous water. (The quantity of heat required to convert one mole of liquid to gaseous water, phase equilibria is slightly different from the quantity required in the previous experiment. This is what we mean when we say that the enthalpy of vaporization varies with temperature.)
This experiment can be repeated for many temperatures. So long as the temperature is in the range \(\mathrm{273.16}
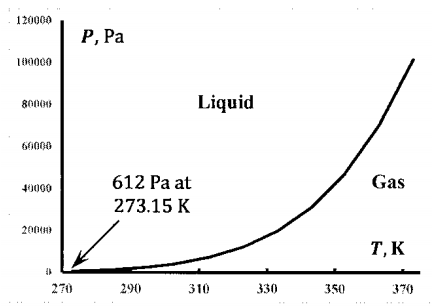
Below \(273.16\ \mathrm{K}\), an equilibrium system containing only liquid and gaseous water cannot exist. At high pressures, a two-phase equilibrium system contains solid and liquid; at sufficiently low pressures, it contains solid and gas. Above \(647.1\ \mathrm{K}\), the distinction between liquid and gaseous water vanishes. The water exists as a single dense phase. This is the critical temperature. Above the critical temperature, there is a single fluid phase at any pressure.
If we keep the pressure constant and remove heat from a quantity of liquid water, the temperature decreases until we eventually reach a temperature at which the water begins to freeze to ice. At this point, water and ice are in equilibrium. Further removal of heat does not decrease the temperature of the water–ice system; rather, the temperature remains constant and additional water freezes into ice. Only when all of the liquid has frozen does further removal of heat cause a further decrease in the temperature of the system. When we repeat this experiment at a series of temperatures, we find a continuous line of pressure–temperature points that are liquid–ice equilibrium points.
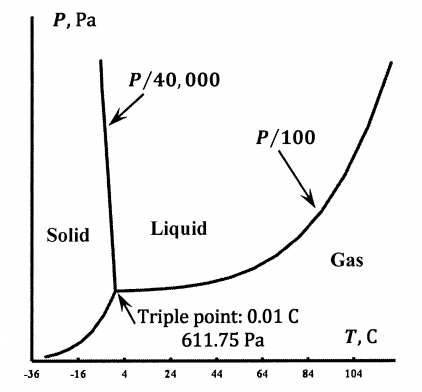
As sketched in Figure 4, the liquid–ice equilibrium line intersects the liquid–vapor equilibrium line. At this intersection, liquid water, ice, and water vapor are all in equilibrium with one another. There is only one such point. It is called the triple point of water. The ice point or melting point of water is the temperature at which solid and liquid water are in equilibrium at one atmosphere in the presence of air. The water contains dissolved air. The triple point occurs in a pure-water system; it is the temperature and pressure at which gaseous, liquid, and solid water are in equilibrium. By definition, the triple point temperature is 273.16 K. Experimentally, the pressure at the triple point is 611 Pa. Experimentally, the melting point is 273.15 K.
To freeze a liquid, we must remove heat. To fuse (melt) the same amount of the solid at the same temperature and pressure, we must add the same amount of heat. This heat is called the latent heat of fusion or the enthalpy of fusion. The enthalpy of fusion for one mole of a substance is usually denoted \({\Delta }_{fus}H\). It varies slightly with temperature and pressure. Tables usually give experimental values of the equilibrium melting temperature at a pressure of 1 bar or 1 atm; then they give the enthalpy of fusion at this temperature and pressure.
At low pressures and temperatures, ice is in equilibrium with gaseous water. A continuous line of pressure–temperature points represents the conditions under which the system contains only ice and water vapor. As the temperature increases, the ice–vapor equilibrium line ends at the triple point. The conversion of a solid directly into its vapor is called sublimation. To sublime a solid to its vapor requires the addition of heat. This heat is called the latent heat of sublimation or the enthalpy of sublimation. The enthalpy of sublimation for one mole of a substance is usually denoted \({\Delta }_{sub}H\). It varies slightly with temperature and pressure.


