8.8: Non-ideality - Henry's Law and Azeotropes
- Page ID
- 84538
The proceeding discussion was based on the behaviors of ideal solutions of volatile compounds, and for which both compounds follow Raoult’s Law. Henry’s Law can be used to describe these deviations.
\[ p_B = k_H p_B^o \nonumber \]
For which the Henry’s Law constant (\(k_H\)) is determined for the specific compound. Henry’s Law is often used to describe the solubilities of gases in liquids. The relationship to Raoult’s Law is summarized in Figure \(\PageIndex{1}\).
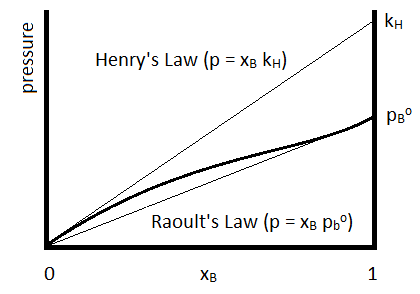
Henry’s Law is depicted by the upper straight line and Raoult’s Law by the lower.
The solubility of \(CO_2(g)\) in water at 25 oC is 3.32 x 10-2 M with a partial pressure of \(CO_2\) over the solution of 1 bar. Assuming the density of a saturated solution to be 1 kg/L, calculate the Henry’s Law constant for \(CO_2\).
Solution
In one L of solution, there is 1000 g of water (assuming the mass of CO2 dissolved is negligible.)
\[ (1000 \,g) \left( \dfrac{1\, mol}{18.02\,g} \right) = 55\, mol\, H_2O \nonumber \]
The solubility of \(CO_2\) can be used to find the number of moles of \(CO_2\) dissolved in 1 L of solution also:
\[ \dfrac{3.32 \times 10^{-2} mol}{L} \cdot 1 \,L = 3.32 \times 10^{-2} mol\, CO_2 \nonumber \]
and so the mol fraction of \(CO_2\) is
\[ \chi_b = \dfrac{3.32 \times 10^{-2} mol}{55.5 \, mol} = 5.98 \times 10^{-4} \nonumber \]
And so
\[10^5\, Pa = 5.98 \times 10^{-4} k_H \nonumber \]
or
\[ k_H = 1.67 \times 10^9\, Pa \nonumber \]
Azeotropes
An azeotrope is defined as the common composition of vapor and liquid when they have the same composition.
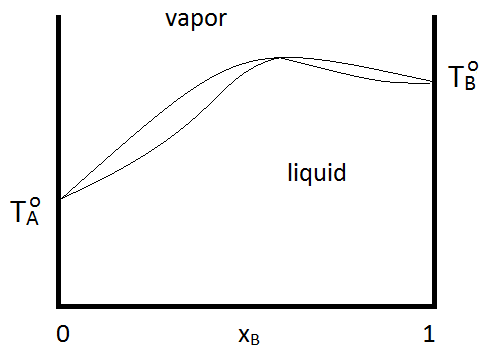
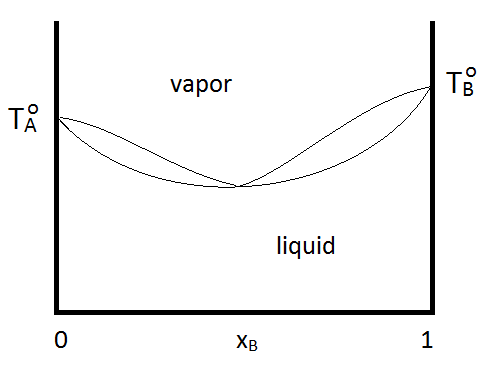
Azeotropes can be either maximum boiling or minimum boiling, as show in Figure \(\PageIndex{2; left}\). Regardless, distillation cannot purify past the azeotrope point, since the vapor and quid phases have the same composition. If a system forms a minimum boiling azeotrope and also has a range of compositions and temperatures at which two liquid phases exist, the phase diagram might look like Figure \(\PageIndex{2; right}\):
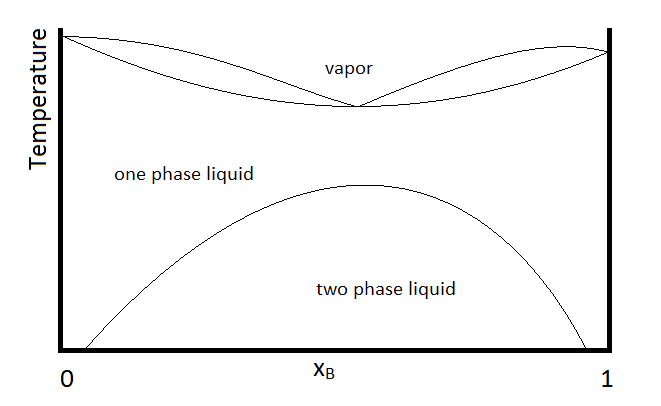
Another possibility that is common is for two substances to form a two-phase liquid, form a minimum boiling azeotrope, but for the azeotrope to boil at a temperature below which the two liquid phases become miscible. In this case, the phase diagram will look like Figure \(\PageIndex{3}\).
In the diagram, make up of a system in each region is summarized below the diagram. The point e indicates the azeotrope composition and boiling temperature.
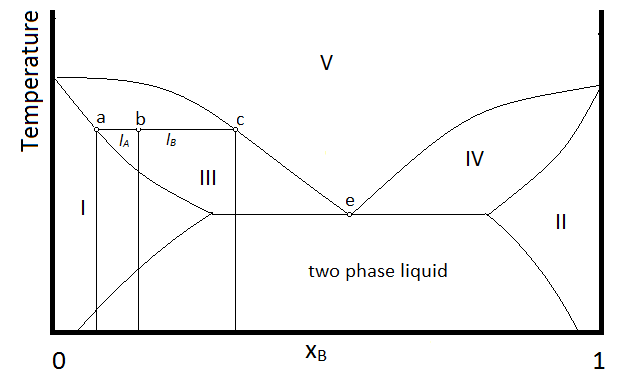
- Single phase liquid (mostly compound A)
- Single phase liquid (mostly compound B)
- Single phase liquid (mostly A) and vapor
- Single phase liquid (mostly B) and vapor
- Vapor (miscible at all mole fractions since it is a gas)
Solution
Within each two-phase region (III, IV, and the two-phase liquid region, the lever rule will apply to describe the composition of each phase present. So, for example, the system with the composition and temperature represented by point b (a single-phase liquid which is mostly compound A, designated by the composition at point a, and vapor with a composition designated by that at point c), will be described by the lever rule using the lengths of tie lines lA and lB.


