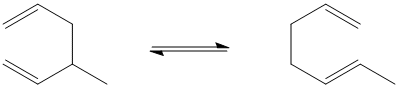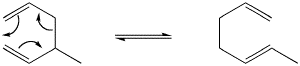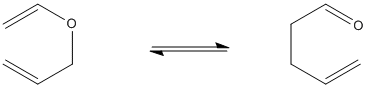PR2. Cope and Claisen Rearrangements
- Page ID
- 4342
A rearrangement is a reaction in which one molecule undergoes bonding changes, with the transfer of one atom or group from one position in the molecule to another.
Proton tautomerism is a kind of rearrangement. A proton is removed from one site in the molecule and put back in a different site nearby. Tautomerism generally requires a couple of proton transfer steps in a row. A proton is removed from one site and then a proton is placed on the new site. (In another variation, a proton is added at one site and then a proton is removed from the old site.)
However, rearrangements often involve the concerted transfer of a group from one site to another within the molecule. The group loses its bond to one site and gains its bond to the other site at the same time.
A Cope rearrangement happens that way.
Figure PR2.1. A Cope rearrangement
At first it may not seem like much has happened in this reaction. The two pi bonds have changed position, however, and so has one of the sigma bonds. That means a total of six electrons have moved (two electrons per bond).
Figure PR2.2. A diagram of electron movement in a Cope rearrangement.
It does not matter which directions you draw the arrows moving in figure PR2.2. They could be shown going clockwise or counterclockwise. There is no electrophile or nucleophile. However, the arrows help with "electron book-keeping." The number of electrons is significant, however.
- Six electrons move in a circle of six atoms.
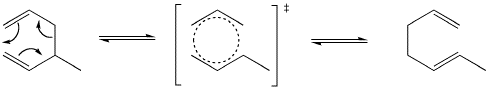
That patterns is reminiscent of benzene. This reaction may be related to the unusual stability of benzene. The transition state for this reaction is considered to be somewhat like benzene. Halfway between one structure and the other, the electrons are delocalized around the ring of atoms.
Figure PR2.3. The transition state in a Cope rearrangement.
A Cope rearrangement can be considered to occur via a rearrangement of overlap between a group of orbitals around this ring. Two orbitals forming a sigma bond tilt away from each other while two orbitals that are pi bonding tilt toward each other.
Figure PR2.4. Orbital rearrangement in a Cope rearrangement
Now there are p orbitals parallel to each other on the left, able to form new pi bonds.
Many concerted rearrangements can be thought of in terms of these orbital reorganizations.
Problem PR2.1.
A Claisen rearrangement is very similar to a Cope rearrangement, but oxygen is involved.
- Draw curved arrows to keep track of electrons in this Claisen rearrangement.
- Draw the aromatic transition state of the Claisen rearrangement.
- Draw the orbital reorganization in the Claisen rearrangement.