Preparation of Unsymmetrical Biaryls by the Diazo Reaction and the Nitrosoacetylamine Reaction
- Page ID
- 66479
Preparation of Unsymmetrical Biaryls by the Diazo Reaction and the Nitrosoacetylamine reaction is an aryl-aryl coupling reaction via a diazonium salt. It is also known as the Gomberg–Bachmann reaction.
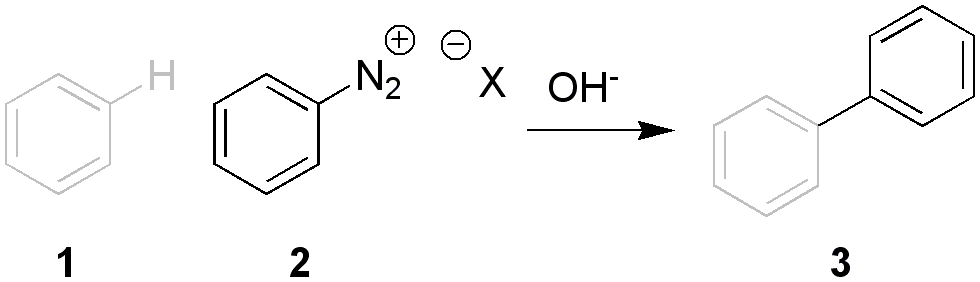
V8rik at the English language Wikipedia [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons
The arene compound 1 (here benzene) is coupled with base with the diazonium salt 2 to the biaryl 3 through an intermediate aryl radical. For example, p-bromobiphenyl may be prepared from 4-bromoaniline and benzene:
BrC6H4NH2 + C6H6 → BrC6H4−C6H5
The reaction offers a wide scope for both diazonium component and arene component but yields are generally low following the original procedure (less than 40%), given the many side-reactions of diazonium salts. Several improvements have been suggested. One possibility is to employ diazonium tetrafluoroborates in arene solvent together with a phase-transfer catalyst, another is to use 1-aryl-3,3-dialkyltriazenes.
Pschorr reaction
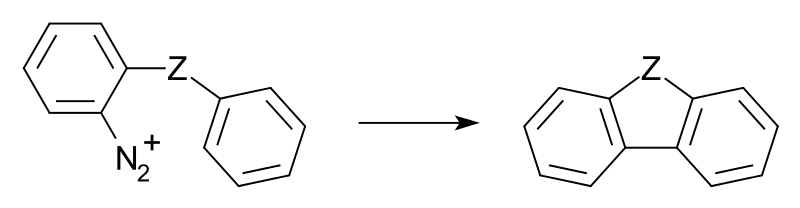
One intramolecular variation which gives better results is the Pschorr reaction:
V8rik at the English language Wikipedia [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons
The group Z can be CH2, CH2CH2, NH and CO (to fluorenone) to name just a few.
See also
- Graebe–Ullmann synthesis
- Meerwein arylation
- Sandmeyer reaction
References
- Gomberg, M.; Bachmann, W. E. (1924). "The Synthesis of Biaryl Compounds by Means of the Diazo Reaction". J. Am. Chem. Soc. 42 (10): 2339–2343. doi:10.1021/ja01675a026.
- W. Pötsch. Lexikon bedeutender Chemiker (VEB Bibliographisches Institut Leipzig, 1989) (ISBN 3817110553)
- M. B. Smith, J. March. March's Advanced Organic Chemistry (Wiley, 2001) (ISBN 0-471-58589-0)
- M. Gomberg and W. E. Bachmann (1941). "p-Bromobiphenyl". Org. Synth.; Coll. Vol., 1, p. 113
- J.R. Beadle, S.H. Korzeniowski, D.E. Rosenberg, B.J. Garcia-Slanga, G.W. Gokel; Korzeniowski; Rosenberg; Garcia-Slanga; Gokel (1984). "Phase-transfer-catalyzed Gomberg-Bachmann synthesis of unsymmetrical biarenes: a survey of catalysts and substrates". J. Org. Chem. 49 (9): 1594–603. doi:10.1021/jo00183a021.
- T.B. Patrick, R.P. Willaredt, D.J. DeGonia; Willaredt; Degonia (1985). "Synthesis of biaryls from aryltriazenes". J. Org. Chem. 50 (13): 2232–2235. doi:10.1021/jo00213a007.
- R. Pschorr (1896). "Neue Synthese des Phenanthrens und seiner Derivate". Chem. Ber. 29: 496. doi:10.1002/cber.18960290198.
- March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- Review Article, Kenneth K. Laali and Mohammadreza Shokouhimehr, The Pschorr Reaction, a Fresh Look at a Classical Transformation Current Organic Synthesis, 2009, 6, 193–202. doi:10.2174/157017909788167275
- Stephen A. Chandler; Peter Hanson; Alec B. Taylor; Paul H. Walton; Allan W. Timms (2001). "Sandmeyer reactions. Part 5.1 Estimation of the rates of 1,5-aryl/aryl radical translocation and cyclisation during Pschorr fluorenone synthesis with a comparative analysis of reaction energetics". J. Chem. Soc., Perkin Trans. 2 (2): 214–228. doi:10.1039/b006184k.
Contributors
- Wikipedia (CC-BY-SA-3.0)