3.5: Solutions to Selected Problems
- Page ID
- 238869
KP5. Solutions to Selected Problems
Problem KP1.1.
Two C-C σ bonds and one new π bond are made. Three old π bonds are lost.
ΔH = bonds broken - bonds made
ΔH = (3 x 64) - ((2 x 83) + 64) kcal/mol
ΔH = -38 kcal/mol
Problem KP1.2.
Tc = ΔH / (ΔS + Rlog[M])
Tc = - 7,000 cal mol-1 (-8.6 cal K-1 mol-1 + 1.98 cal K-1 mol-1 log(8.7))
Tc = - 7,000 (-8.6 + 1.98 (0.939)) K
Tc = - 7,000 (-6.74) K
Tc = 1038 K = 765 °C
Problem KP1.3.
For each amide bond, a C-N bond and a H-Cl bond are made. A C-Cl and a N-H bond are lost.
ΔH = bonds broken - bonds made
= (C-Cl + N-H) - (C-N + H-Cl)
ΔH = (81 + 93) - (73 + 102) kcal/mol
ΔH = -1 kcal/mol
Problem KP2.1.
75% conversion means 0.75 in terms of fractions.
DP = 1 / (1 - p)
DP = 1 / (1 - 0.75)
DP = 1 / 0.25
DP = 4
Problem KP2.2.
slope = 2 [M]0 k = 0.717 s-1
k = 0.717 s-1 / (2 x 17 mol L-1)
k = 0.021 L mol-1s-1
Problem KP2.3.
Mn = M0 / (1 - p)
= 120 g/mol / (1 - 0.99)
= 120 g/mol / 0.01
= 12,000 g/mol
Mw = M0(1 + p) / (1 - p)
= 120 g/mol (1 + 0.99) / ( 1 - 0.99)
= 120 g/mol (1.99) / 0.01
= 23,800 g/mol
D = 1 + p
= 1 + 0.99
= 1.99
Problem KP3.1.
Rate = k' [M][I]1/2
slope = k' [I]1/2
0.0024136 s = k' (0.00025)1/2
k' = 0.015 s-1
Problem KP3.2.
At steady state:
Rateinit = Rateterm
ki [M][I] = kt[M+]
Rearranging:
[M+] = (ki/kt) [M][I]
Problem KP3.3.
Rateprop = kp [M+][M]
Substituting the steady state expression for [M+]:
Rate = (kikp/kt) [M]2[I]
Problem KP3.4.
v = Rateprop/Rateinit
v = kp [M+][M] / ki [M][I] = (kp / ki)[M+] / [I]
but [M+] is not a known quantity. Alternatively, at steady state, Rateinit = Rateterm
v = Rateprop/Rateterm
v = kp [M+][M] / kt [M+] = (kp / kt)[M]
Problem KP3.5.
a) v = [M]0/[I]0 = 4.5 / 1.25 x 10-3 = 3,400
b) v = (kp/2f kt kd)([M]/[I]1/2)
v = (0.003 / 2(0.5)(0.003)(0.0001))(4.5/(1.25 x 10-3)1/2) = (1/0.0001)(4.5/0.035) = 128/0.0001 = 1,280,000
c) v = (kp/2f kt kd)([M]/[I]1/2)
v = (0.003 / 2(0.5)(0.03)(0.0001))(4.5/(1.25 x 10-3)1/2) = (0.01/0.0001)(4.5/0.035) = 128/0.01 = 12,800
d) v = (kp/2f kt kd)([M]/[I]1/2)
v = (0.003 / 2(0.5)(0.003)(0.1))(4.5/(1.25 x 10-3)1/2) = (1/0.1)(4.5/0.035) = 128/0.1 = 1,280
Problem KP4.1.
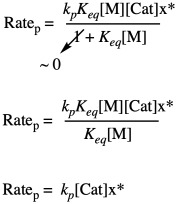
The key point is that, when two terms are added together and one is much larger than the other, the sum is approximately the same as the larger of the two terms. You can ignore the smaller one.
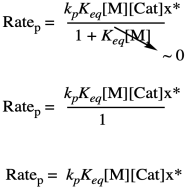
Problem KP4.2.