2.7: Living Anionic Polymerization
- Page ID
- 238856
Unwanted side reactions in anionic polymerization, such as back-biting or Claisen reactions with acrylate chains, lead to early chain death and a broadening of the molecular weight distribution. This problem is intrinsic to polymer growth. Because reactive chain ends are needed to enchain additional monomers, there is always the potential that these relatively high-energy species will go off track and lead to different products.
Living polymerization describes any system in which early chain death is limited, so that polymer chains can continue to grow uniformly. In these systems, the molecular weight increases linearly with the percent conversion of monomer to polymer. In addition, dispersity remains low even at high percent conversion.
The reactive chain ends in anionic polymerizations are nucleophilic carbon anions. If you have studied these kinds of compounds before, the idea of covalency might come to mind. Carbon anions are easier to work with if they are not really anions, but instead share their electrons with their counterions to some degree. So, for example, we might choose to employ lithium counterions with these anionic chain ends, rather than sodium or potassium. The smaller, more electronegative lithium (at least compared to sodium or potassium) can form a polar covalent bond with carbon, stabilizing the nucleophile.

Of course, even an alkyllithium is a strong enough nucleophile to initiate anionic polymerization, provided the resulting anion is more stable than the initial one. In general, it can initiate the formation of growing chains if the resulting anion is delocalized.
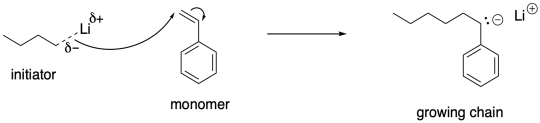
We can think of the growing chains as being in equilibrium between having covalent lithium-carbon bonds and forming ion pairs. The ion pair would be more ready to react with the next monomer. That equilibrium could form a basis for a dormant state and a growing state. Just as in living cationic polymerization, the growing state is necessary for polymer chain growth but is susceptible to unwanted side reactions. The dormant state protects the growing chain by limiting the growing chain concentration, consequently limiting the degree of side reactions.
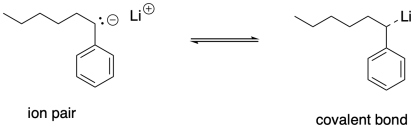
So, just using a lithium counterion, for instance, might be expected to promote living polymerization, keeping dispersity low. For that reason, it may be surprising that one of the strategies used for chain control in anionic polymerizations is to add potassium alkoxides along with the alkyllithium initiator. If lithium bases have greater covalency and offer greater control, why would you add potassium bases?
That question has even more merit if you explore the history of mixed-metal bases. Schlosser's base is a well-precedented example. Typically, it's a mixture of butylllithium and potassium tert-butoxide. Developed by Manfred Schlosser at EPF (ETH) Lausanne in Switzerland, mixtures of alkylithiums and potassium alkoxides form powerful bases capable of deprotonating hydrocarbons such as toluene. The mechanism of achieving such high base strength is believed to involve transfer of an alkyl anion from lithium to potassium. From the point of view of making growing chains more covalent, providing a dormant state, this doesn't seem like a good idea. Nevertheless, it works. How?
One of the other features of these mixtures (Schlosser called them LiCKOR bases, noting the mix of lithium and potassium components) is a high level of aggregation. Aggregates are clusters of molecules that stick together. For Schlosser's base, the simplext aggregate would be one alkylithium molecule bound to one potassium tert-butoxide molecule.
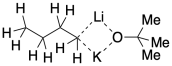
What holds aggregates like this together? The anions can bridge between alkali metals. With the alkoxide ion, that's easy to imagine: the oxygen atom has more than one lone pair, so it can donate one to lithium and one to potassium. It's a little harder to see how the alkyl anion, with only one lone pair, could do that. However, that sort of interaction in which one lone pair is shared between two or more lithium ions, although rare, is pretty well-documented in some alkyllithiums. It's like the alkyl anion has been caught midway between two lithiums, transferring from one to another.
Bigger aggregates could form if additional molecules stuck together. We can easily picture this happening if one alkyllithium combined with two potassium alkoxides.
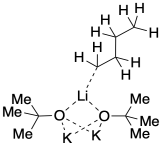
You can probably imagine even larger aggregates. Maybe two alkoxides come together with one alkyllithium, held together by bridging oxygens. In fact, these structures seem to be very dynamic. They can come apart in solution, and they can come together to make even bigger structures. In reality, a number of different aggregation states will exist in equilibrium with each other, and some might contain eight or twelve alkali cations together with their accompanying anions.
So, what is the role of aggregation in producing a dormant state? It may cap the end of the growing chain temporarily, so that the anionic chain end is less likely to interact with monomers. Reaction would occur only when the aggregate broke up, freeing an anionic chain end.
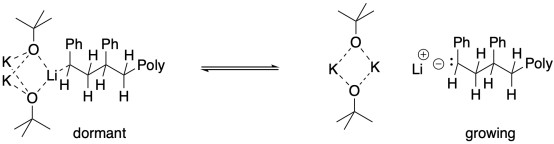
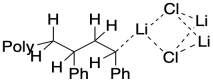
Aggregate formation can also be promoted by other anions, including simple halides such as chloride and fluoride. As a result, the addition of simple lithium salts can be effectine in promoting living anionic polymerization. The alkoxide base needn't play a role.
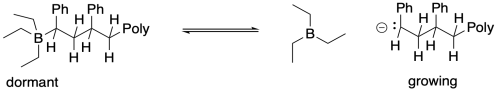
An alternative strategy for living anionic polymerization involves the addition of Lewis acidic compounds as chain control agents. In these cases, the equilibrium between dormant and growing chains would involve coordination of the anionic chain end to the Lewis acidic atom. Because Lewis acid-base complexes occur in equilibrium, some fraction of the polymers would always exist in the growing phase, but a greater fraction would always be found in the dormant phase.
Exercise 2.7.1
Rank the following ions in terms of covalency with oxygen (most covalent to least covalent).
- Na+, Li+, K+
- Mg2+, Ca2+, Be2+
Exercise 2.7.2
Coordination number can vary with the size of a cation. Rank the following ions from largest to smallest.
- Na+, Li+, K+
- Mg2+, Ca2+, Be2+
Exercise 2.7.3
Which compounds would be expected to stabilize growing anionic chains?
- Et3N or Et3Al
- Et2Zn or Et2O
- Ph3B or Ph3N
- (CH3O)2AlCH3 or (CH3O)2CHCH3


