21.4: Electrophilicity of Carbonyl Groups Versus Carboxyl Groups
- Page ID
- 216883
In terms of electrophilic character, carboxyl groups are not as reactive as carbonyl groups. Examination of the resonance structures reveals that the carbonyl carbon bears a higher degree of positive charge than the carboxyl carbon, and is therefore a better (more reactive) electrophile.
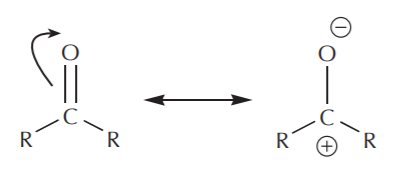
carbonyl group
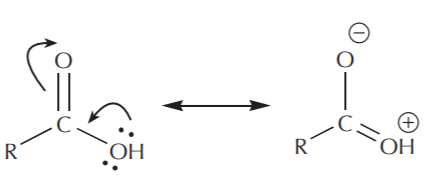
carboxyl group
Although the above example uses a carboxylic acid as the instance of carboxyl group, acid chlorides and esters behave similarly. You should be able to draw the resonance structures for both of these groups as well.
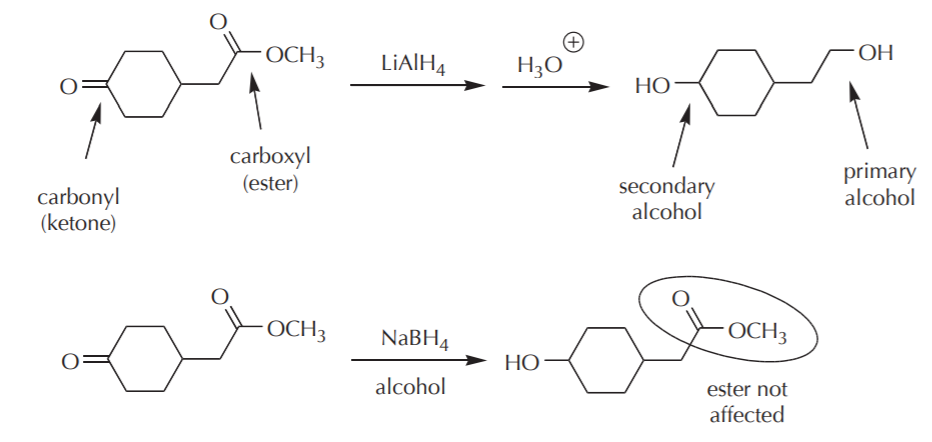
The difference in reactivity between the two groups means that the carbonyl group can be reduced with both high reactivity reducing agents such as lithium aluminum hydride, and less reactive agents such as sodium borohydride. The carboxyl group, on the other hand, will respond only to lithium aluminum hydride and will not be affected by sodium borohydride. This is illustrated by the following example.