21.1: Using Hydrogen as a Nucleophile in Hydride Reductions
- Page ID
- 216857
Like carbon, hydrogen can be used as a nucleophile if it is bonded to a metal in such a way that the electron density balance favors the hydrogen side. A hydrogen atom that carries a net negative charge and bears a pair of unshared electrons is called a hydride ion. How much negative charge density resides on hydrogen depends on the difference in electronegativity between hydrogen and the metal it’s bonded to.
The following table shows the difference in electronegativity between hydrogen and some common metals. Obviously, the greater the difference in electronegativity, the greater the density of negative charge on hydrogen, and the greater the reactivity of the compound as a hydride delivering agent.

For many routine synthetic purposes, sodium and lithium hydrides are simply too reactive, requiring special handling such as inert atmosphere, and careful control of reaction conditions. Calcium hydride is more manageable because it is less reactive, and it is preferred in many reactions. However, many reductions of organic compounds such as carbonyl and carboxyl compounds use aluminum and boron hydride reagents.They are manageable in the laboratory, they are commercially available, and they can be modified to fine-tune their reactivity to various degrees for specific uses. Two of the most widely used hydride reagents in organic synthesis are lithium aluminum hydride, and sodium borohydride, shown below.
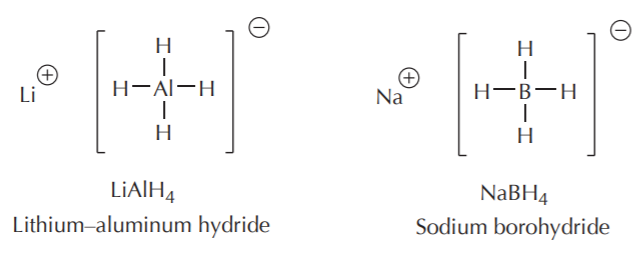
As can be seen from their structure, lithium and sodium are not bonded to hydrogen. They are merely counterions for the negative portion, which is the actual hydride–delivering agent. Second, they are each capable of delivering up to 4 hydride equivalents. Last, we expect sodium borohydride to be less reactive, and therefore more selective, than lithium aluminum hydride. This is in fact the case.
Another way to control the reactivity of these compounds is to replace two hydrogens with bulky alkyl groups, as in the following structures.
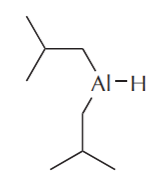 Diisobutylaluminum hydride (DIBAL, or DIBAL-H)
Diisobutylaluminum hydride (DIBAL, or DIBAL-H)
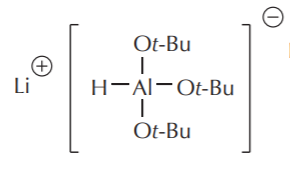 Lithium–tri–t–butoxyaluminum hydride LiAlH(OtBu)3
Lithium–tri–t–butoxyaluminum hydride LiAlH(OtBu)3
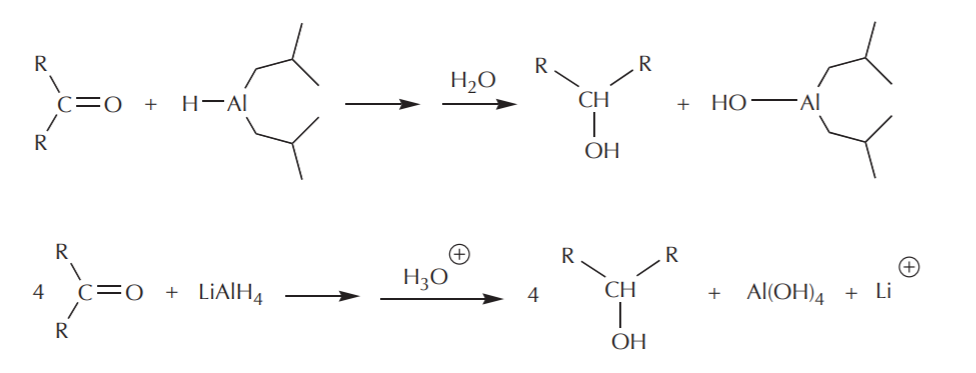
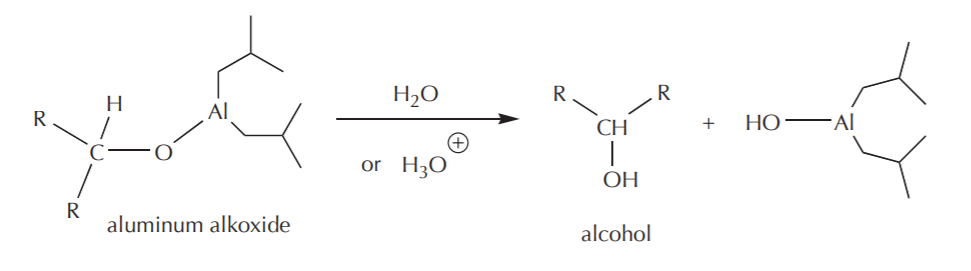
These modifications do two things. The bulky groups prevent fast access of the hydride reagent to the electrophile by a steric effect, and each of them is capable of delivering only one hydride ion instead of four. The mechanism of the hydride attack on a carbonyl carbon shown below demonstrates how these reagents in general work. The hydride-delivering agent must approach the carbonyl carbon until it’s close enough to deliver the hydride ion. At the same time, the pi electrons from the C=O bond move to create a new bond to the metal, forming an alkoxide ion as the product, which is an alcohol functional group equivalent.

The last step towards formation of the alcohol is then protonation of the alkoxide using water or dilute acid. As in the case of Grignard reactions, this step is always assumed, and as such it may or may not be shown explicitly.
Balanced equations reflect the number of hydride ions delivered for complete reduction: