18.3: Use of Carbon Nucleophiles in Organic Synthesis
- Page ID
- 216696
Carbon nucleophiles are widely used in organic synthesis to create new carbon-carbon bonds when they react with electrophiles, and therefore exapand a carbon chain. To be nucleophilic, the carbon atom must be bonded to a less electronegative atom to create a dipole favoring higher electron density on carbon. In most cases this atom is a metal, typically an alkali (group I) or alkaline earth (group II) metal. The difference in electronegativity between the carbon and the metal dictates the degree of nucleophilicity (or basicity) of the carbon atom. Some of the most commonly used metals are Li, Na, K, and Mg. Such species that contain a carbon-metal bond are known as organometallic reagents, or just organometallics, in organic chemistry. Examples are:
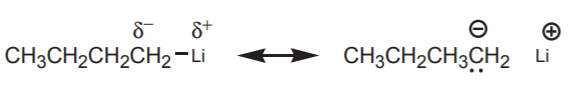 Organolithium reagents
Organolithium reagents
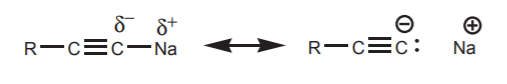 Sodium acetylides
Sodium acetylides
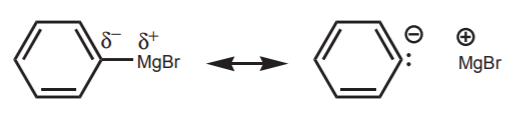 Organomagnesium halides (Grignard reagents)
Organomagnesium halides (Grignard reagents)
Two important types of organometallics used as carbon nuclephiles are the acetylides (ch. 9) and the Grignard reagents (ch. 10). More specifically, we focus on the reactions between these substances and carbonyl compounds (aldehydes and ketones) to produce primary, secondary, and tertiary alcohols. Before we proceed, we must revisit the characteristics of electophiles, since it is a reaction between a nucleophile and an electrophile that we are considering now.


