17.6: Reaction Examples
- Page ID
- 216692
SOLVED PROBLEM 8-1, p. 329

Analysis: Markovnikov addition of HBr to the C=C bond. Use HBr:
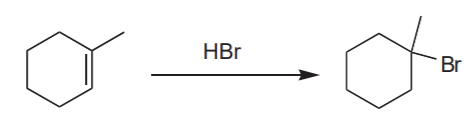
Complement:

Analysis: Anti-Markovnikov addition of HBr to the C=C bond. Use HBr in the presence of peroxides
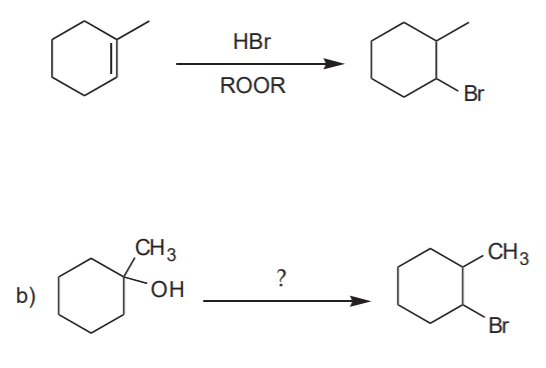
Retrosynthetic analysis, or retrosynthesis:
Recommended problem: 8-4, p. 330
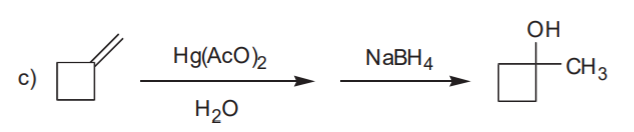
OXYMERCURATION - DEMERCURATION, ALSO CALLED OXYMERCURATION - REDUCTION
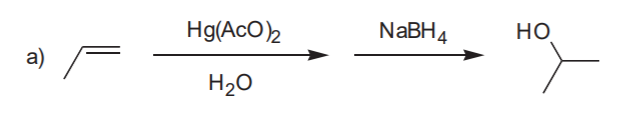 Markovnikov alcohol
Markovnikov alcohol
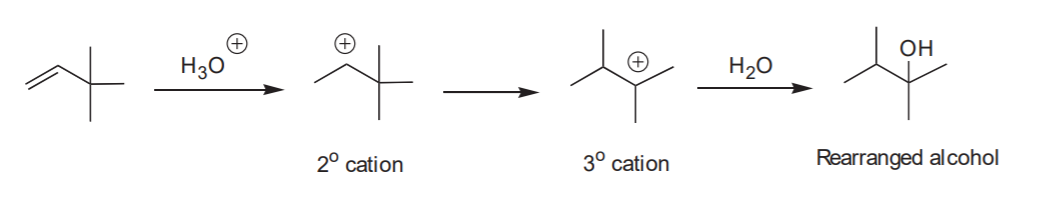
 Markovnikov alcohol without rearrangement
Markovnikov alcohol without rearrangement
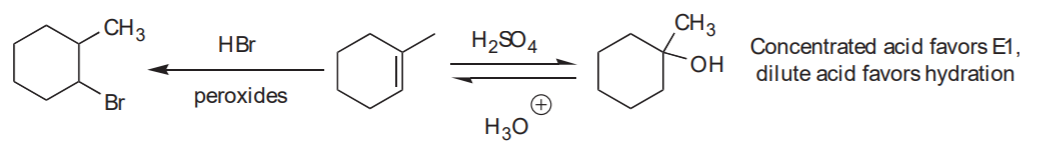
Contrast with addition of water in the presence of strong acid:
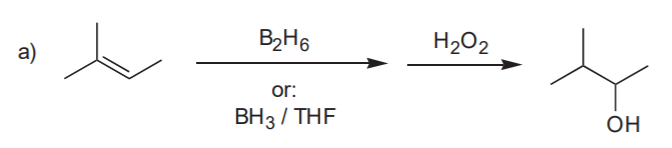
HYDROBORATION - OXIDATION SEQUENCE.
An effective way to make Anti-Markovnikov alcohols. Water adds to the double bond with syn-stereochemistry.
 Anti-Markovnikov alcohol
Anti-Markovnikov alcohol
SOLVED PROBLEM 8-3, p. 339
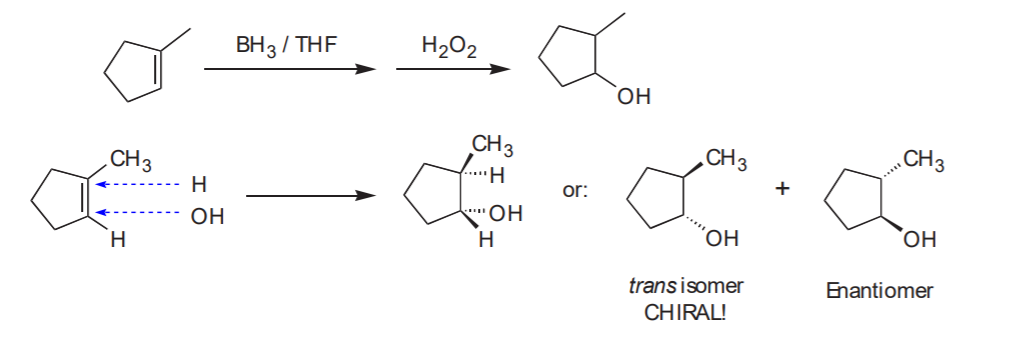
Problem 8-15 (b)
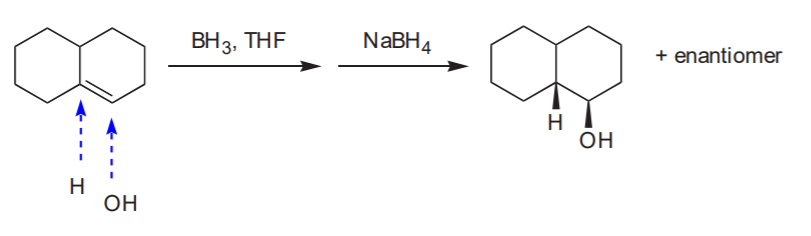
Can I make B from A by hydroboration - oxidation?
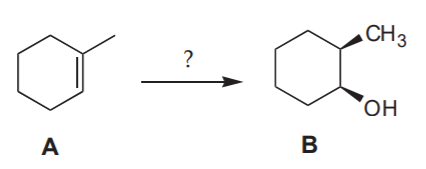 Notice that the methyl and the alcohol group are cis to each other.
Notice that the methyl and the alcohol group are cis to each other.
Analysis:
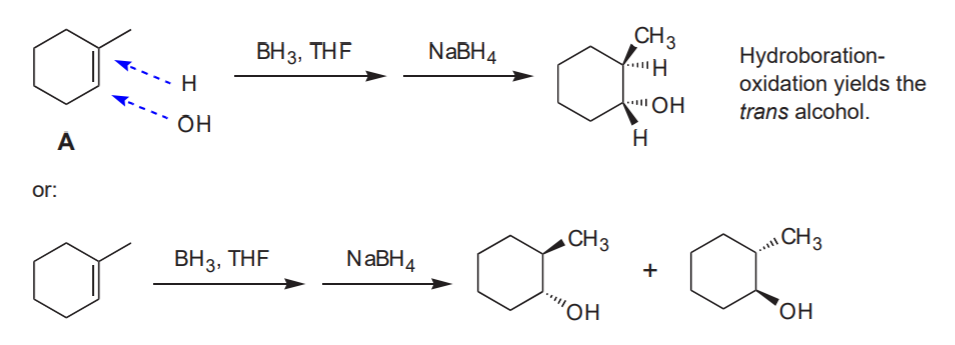
Answer: NO
Recommended: 8-15 (c), p. 342
ADDITION OF CHLORINE OR BROMINE TO THE C=C BOND
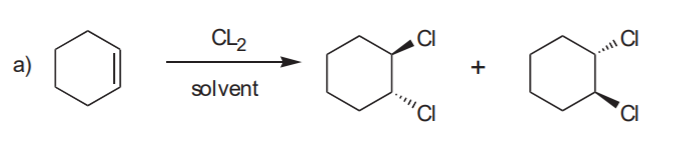
Anti addition yields the trans product, which is chiral. Therefore the enantiomer also forms.
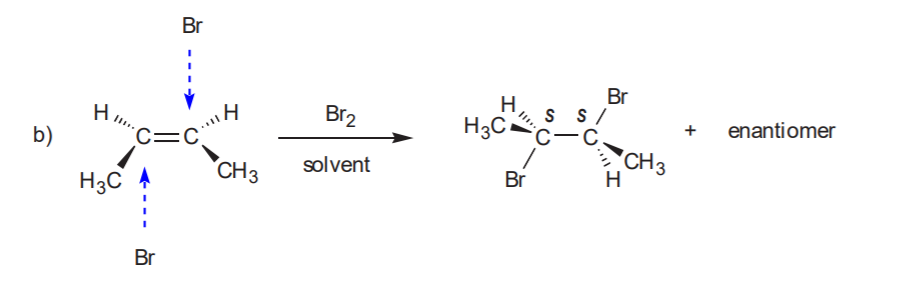
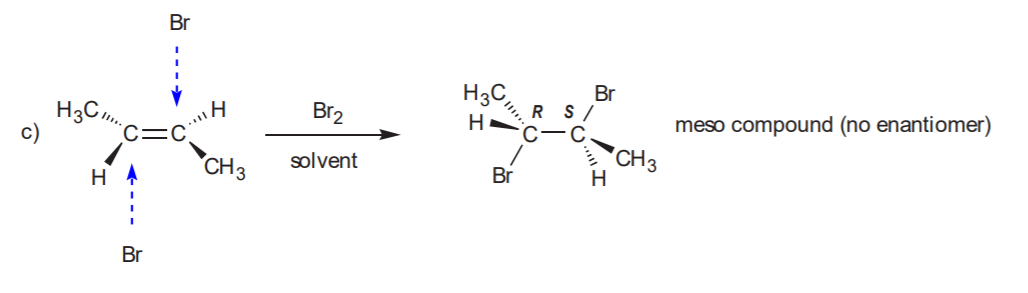
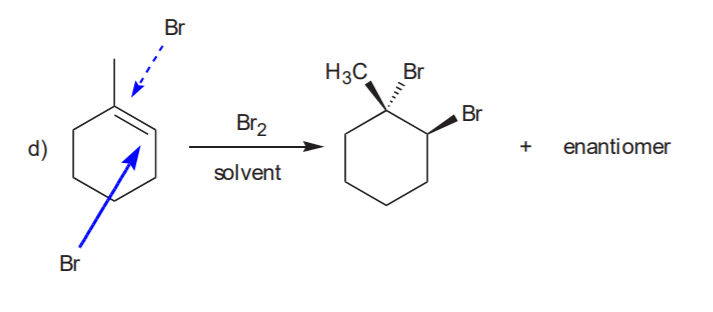
Recommended: 8-17, p. 345
Variation with water:
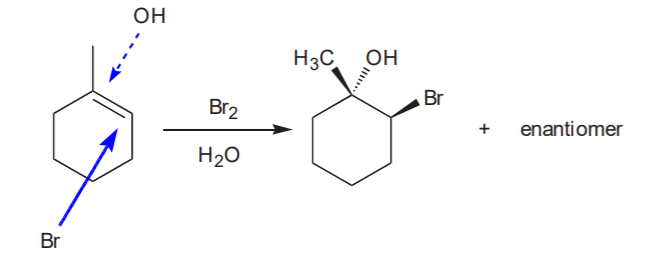
Recommended: 8-5 and 8-6, p. 347
CATALYTIC HYDROGENATION - Transformation of alkenes into alkanes (syn addition of hydrogen).
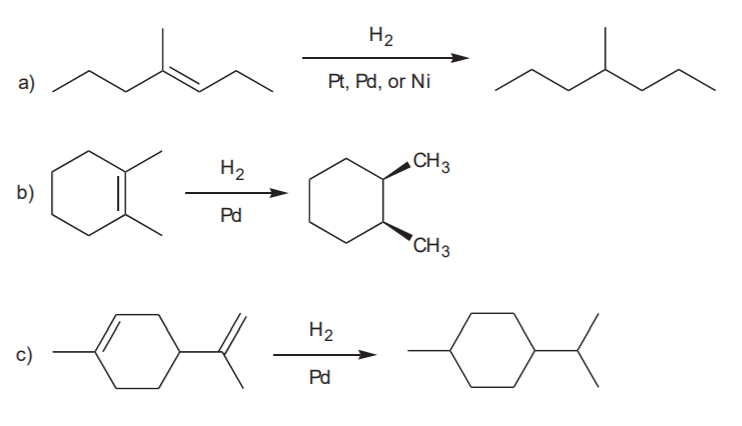
Recommended: 8-23, p. 350
SYN HYDROXYLATION - Syn addition of OH / OH to the C=C bond.
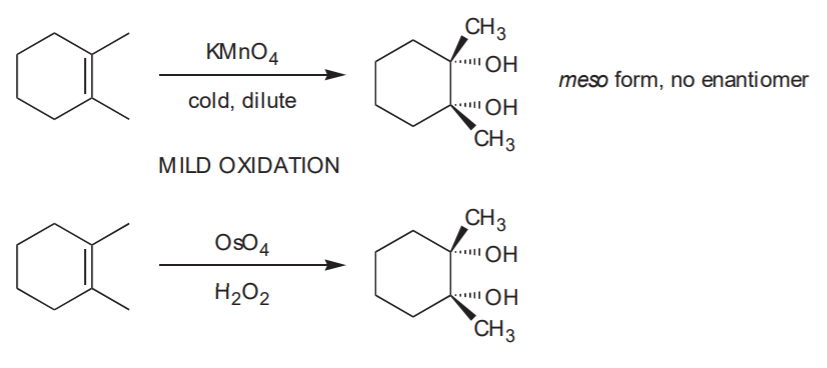
ANTI HYDROXYLATION SEQUENCE- Anti addition of OH / OH to the C=C bond.
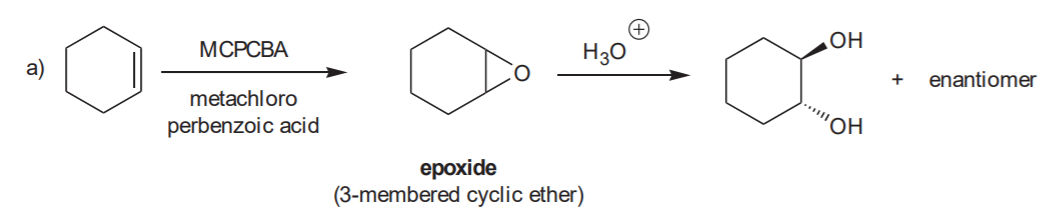
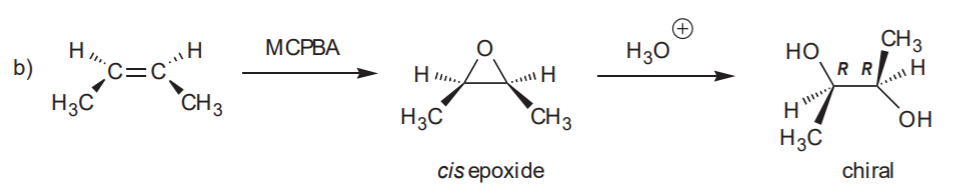
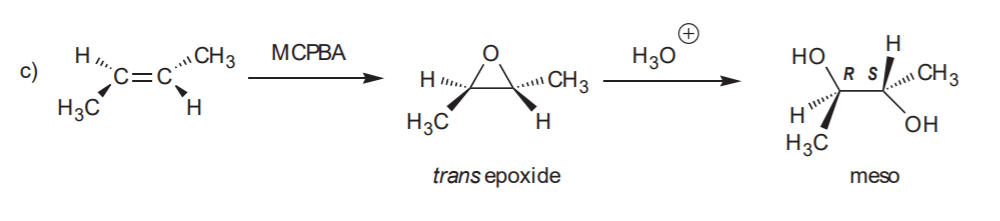
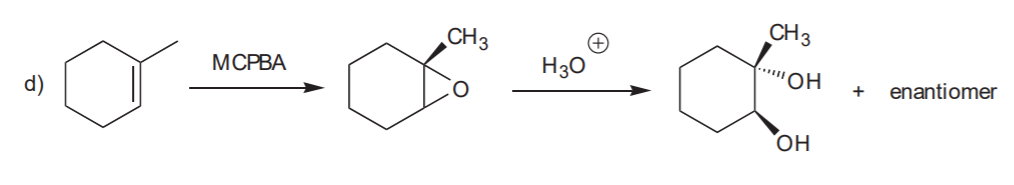
Recommended for syn and anti hydroxilation: 8-34 (all), p. 359
OXIDATIVE CLEAVAGE: Strong oxidation with potassium permanganate.
In this reaction each of the sp2 carbons involved in the pi bond gets oxidized to its maximum possible oxidation state. Refer to notes set # 20 (Oxidation and Reduction in Organic Chemistry) to find out what these states are.
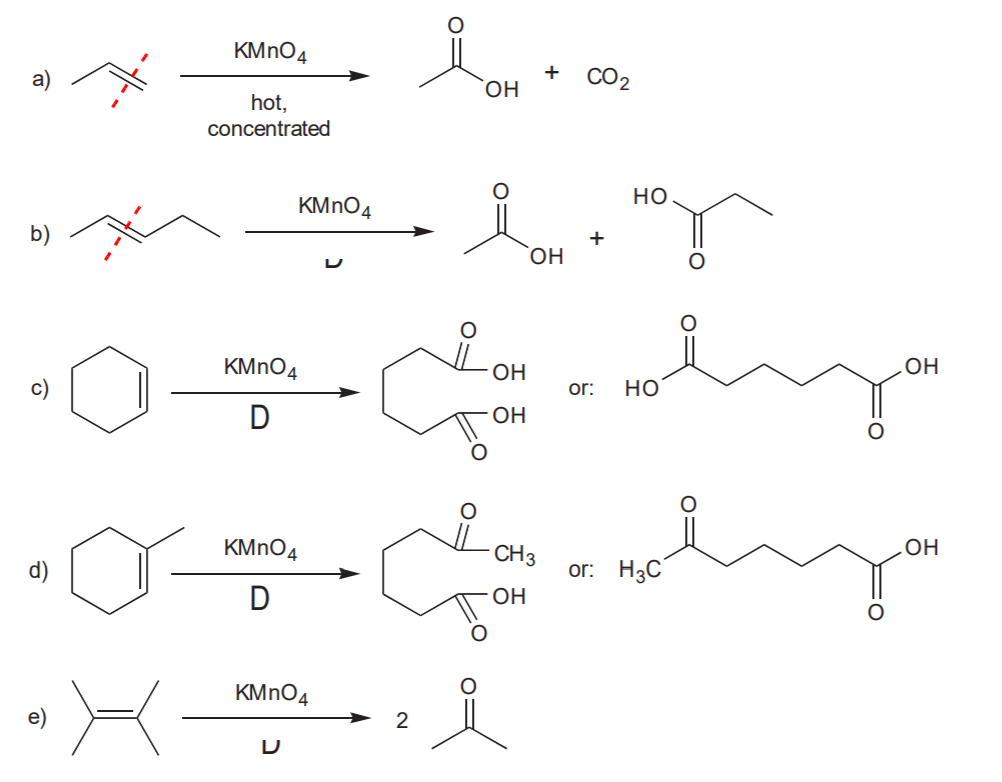
OXIDATIVE CLEAVAGE: Ozonolysis
In this reaction each of the sp2 carbons involved in the pi bond gets oxidized either to aldehyde or ketone, depending on whether it ends up at the end of a carbon chain or in the middle after the pi bond cleaves. If the oxidized carbon ends up at the end of a carbon chain it becomes an aldehyde, otherwise it becomes a ketone.
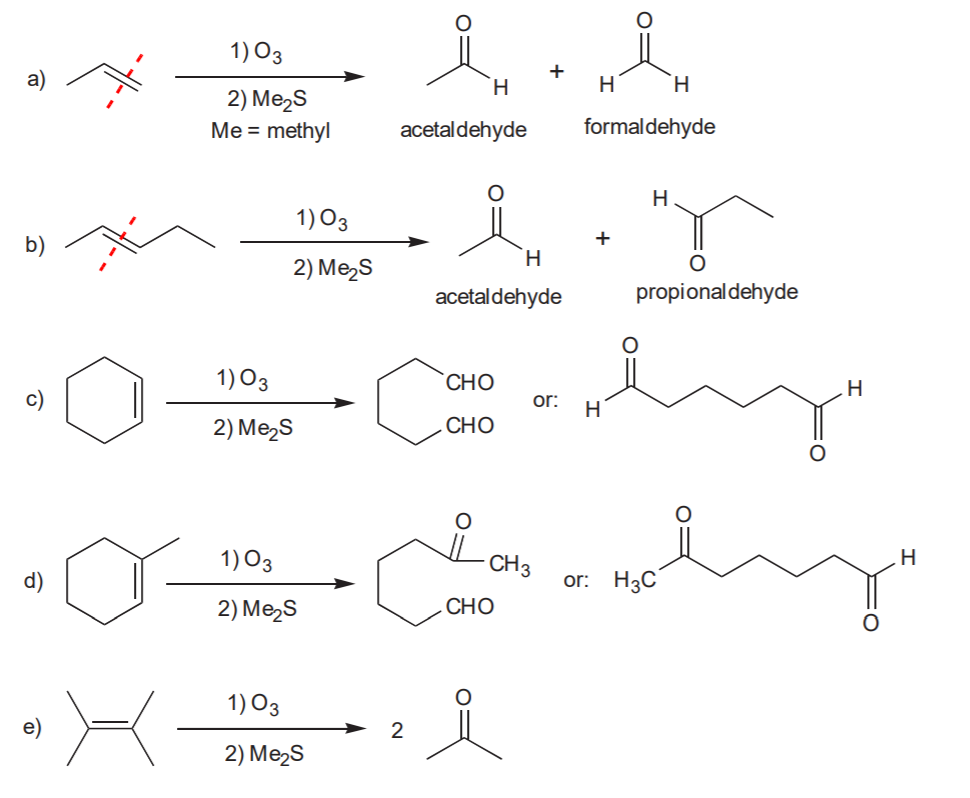
Recommended strong oxidation (oxidative cleavage) with KMnO4 and with ozone: 8-7, 8-36, and 8-37, p. 362-363.
Recommended problems from the end of the chapter: 47 (all), 49 ( a-f ), 58 (all), 63


