17.5: More on the Chemistry of 3-Membered Rings
- Page ID
- 216691
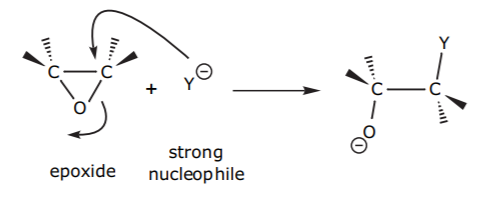
Three-membered rings such as epoxides (cyclic ethers) are strained and susceptible to ring-opening. This can happen for example when a nucleophile attacks one of the ring carbons, breaking the C-O bond. The alkoxide group (oxygen with a negative charge) is normally not a good leaving group, but the driving force for the reaction is release of ring strain.
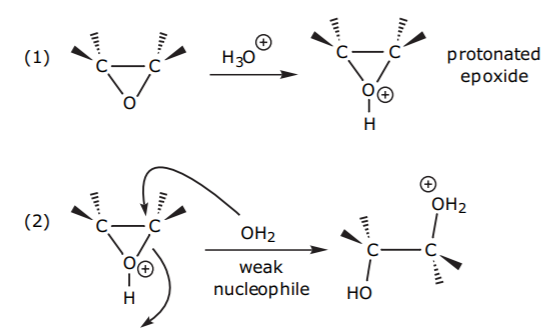
Weak nucleophiles, which may not be strong enough to open the ring, can be assisted by acid catalysis. Protonation of the oxygen with strong acid enhances its leaving group ability, and it also makes the ring carbons more electrophilic (see below).
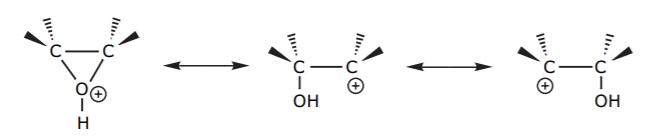
The positive charge in the protonated epoxide is actually shared by the oxygen and the two carbons. The resonance structures show partial ring opening with positive charges on each of the two carbons.
In an unsymmetrical epoxide, the greatest resonance contributor would be the one forming the most stable cation. That is, the one where the positive charge is on the carbon bearing the most alkyl groups.
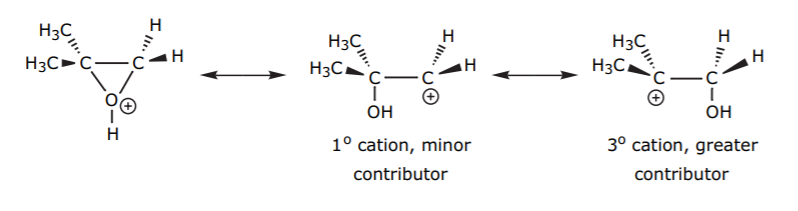
The attack of the nucleophile on the protonated epoxide will therefore take place on the carbon that carries the greater amount of positive charge, even though it is seemingly more sterically hindered. To visualize this, remember that this is not a normal sp3 carbon with an ideal bond angle is 109.5 degrees. Forcing the internal ring angle to a theoretical value of 60 degrees in the epoxide forces the external angle to open up. Also, the resonance structures of the protonated epoxide show that such carbons have substantial sp2 character and therefore are more planar than a regular sp3 carbon.
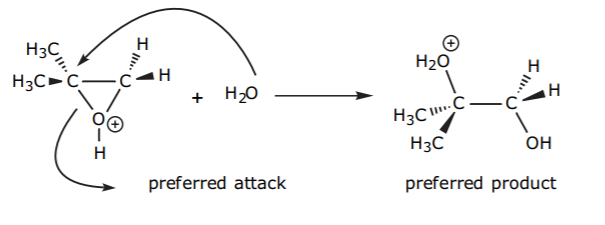
The product then loses a proton (in an acid-base reaction with water, for example) and forms a vicinal dialcohol, or vic diol. The following example shows formation of the trans diol that results from backside attack of water on the epoxide.

Several of the reactions from chapter 8 will further illustrate different aspects of the chemistry of three membered rings to explain the observed products


