15.2: Alkyl Halides as Sn1 and E1 Substrates
- Page ID
- 216053
As mentioned before, conditions that favor Sn1 also favor E1 reactions. The first and rate-determining step in the process is departure of the leaving group to form a carbocation. Let’s look at one of the examples from the previous page, the reaction between 2-bromo-3-methylbutane and methanol. As has been mentioned before, commonly used solvents in Sn1 reactions are water and alcohols. They frequently also double as nucleophiles. In E1 reactions these same substances would act as bases to capture a proton in the elimination step.
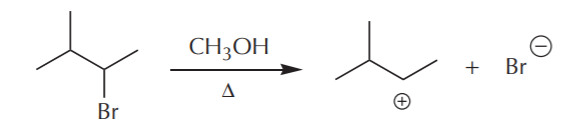
Once formed, the carbocation can:
a) Go on to form the Sn1 product. Remember that when the nucleophile is a neutral protic solvent, its conjugate base replaces the leaving group. This implies that a proton (H+) gets released in the process.

The bromide ion released in the first step and the proton released in the second step can then get together to form HBr, which is an inorganic product of the reaction. However, we will not focus on inorganic products. As a matter of fact, inorganic products are frequently left out when writing organic reactions and mechanisms to avoid clutter and keep the focus on the organic products.
b) Rearrange to a more stable cation. We already mentioned that this can happen by an alkyl or a hydride shift, depending on which process yields a more stable cation. This cation can in turn form another Sn1 product. This Sn1 product is a structural isomer of the one formed in (a) because its connectivity has changed.
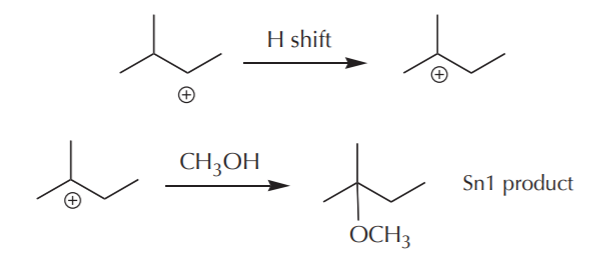
c) Eliminate a proton to form an alkene. Remember that when there is a positive charge on carbon, the neighboring protons become highly acidic. The solvent –acting as a weak base– can capture one of these protons and cause an electron shift towards the positive charge that results in formation of a new pi bond, or alkene product. When the protons surrounding the positive charge are nonequivalent, several alkenes are possible, as illustrated below.
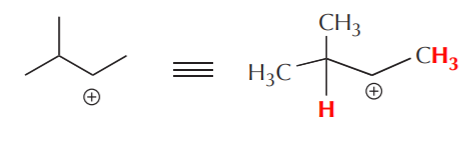
Secondary cation before rearrangement. The protons shown in red are now acidic due to the presence of the positive charge on carbon.
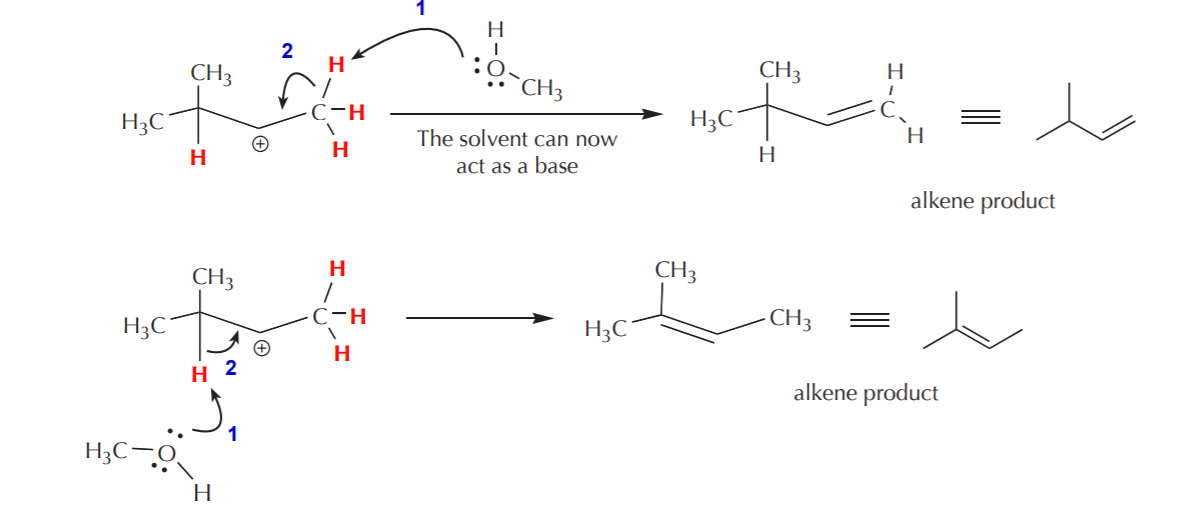
The rearranged cation can undergo similar elimination processes to yield two possible alkenes, but one of them is the same as one of the alkenes formed above, with the pi bond located between carbons 2 and 3. As an exercise, see if you can identify the protons which are acidic, how they are eliminated, and how electron movement takes place to form the alkene structures shown below.
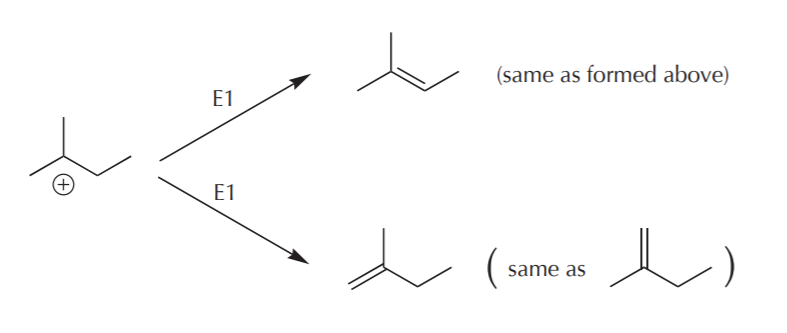
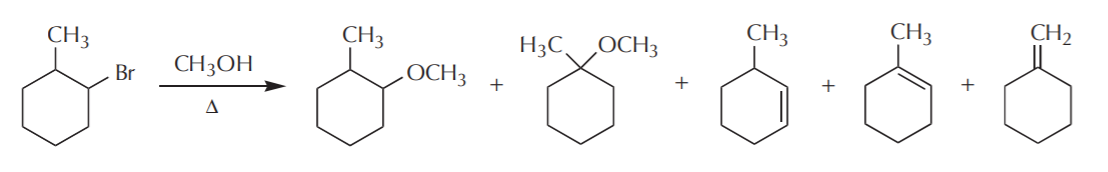
Thus we have the net reaction we introduced on page 1, showing the formation of Sn1 and E1 products:

Can you now explain (mechanistically) how the various products form in the second example given on p.1?