15.4: Eliminations Involving Asymmetrical Substrates
- Page ID
- 216055
In some eliminations the products include several possible alkenes. The next question is then, which ones form preferentially? Is there a rule for predicting which alkene will predominate? Let’s look at the following examples given before, but this time let’s focus only on the alkene products.
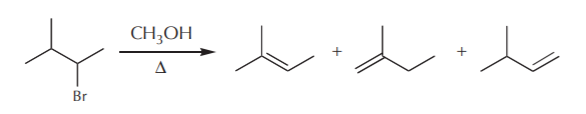
Saytzeff’s rule enables us to make a prediction. According to this rule, in elimination reactions, the most highly substituted alkene usually predominates. For details, refer to the Wade textbook, section 6-19 (5th ed.) or 6-18 (6th ed.).
The most highly substituted alkene is the one with the most alkyl groups directly attached to the carbon-carbon double bond. This of course does not include hydrogens attached to the carbon-carbon double bond. In the above example, we have mono, di, and trisubstituted products. According to Saytzeff’s rule, the trisubstituted product will predominate because it is the most stable.
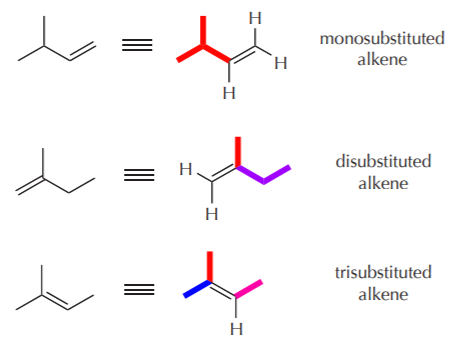
Why is the trisubstituted alkene more stable than the other two? The relative stabilities of alkenes is measured by their heat of hydrogenation (much like the relative stabilities of cycloalkanes is measured by their heats of combustion, see ch. 3). The relationship is inverse: the higher the heat of hydrogenation, the lower the stability, because the higher the heat of hydrogenation, the higher the potential energy of the alkene.
For a full discussion of this trend with tables, refer to the Wade textbook, section 7-7 (A-D) in both the 5th and the 6th eds. Please note that in the case of cis and trans isomers, the higher stability of the trans isomer is due to a steric effect. The alkyl substituents in the cis isomer are closer to each other than in the trans isomer, leading to increased steric crowding.

Let’s look at the last example. In this case we have di and trisubstituted products. Once again, the trisubstituted product will be predominant because it is the most stable.



