15.3: Alcohols As Sn1 and E1 Substrates
- Page ID
- 216054
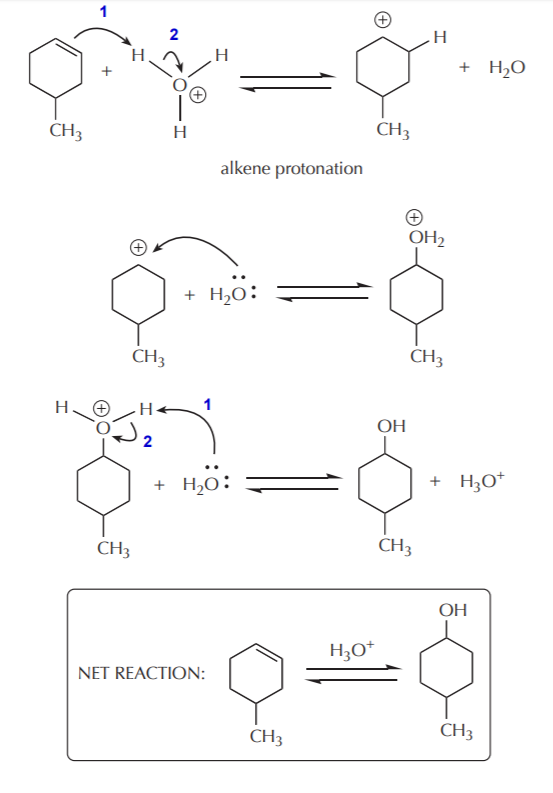
The classic textbook example of E1 elimination reactions is the acid-catalyzed alcohol dehydration. Strong acid catalysis is needed to protonate the hydroxyl group of alcohols and turn it into a good leaving group. For alcohols which are soluble in water, an aqueous solution of strong acid is usually used. Such solution contains a high concentration of hydronium ion (the conjugate acid of water), which acts as the proton donor. This first protonation step is an acid-base reaction, and as such it takes place very rapidly. The protonation of 4-methylcyclohexanol illustrates this step.

Once protonated, the hydroxyl group can leave as water, leaving behind a positively charged carbon. This is the rate determining step in the sequence.

The elimination step takes place after formation of the carbocation, with water acting as a base.
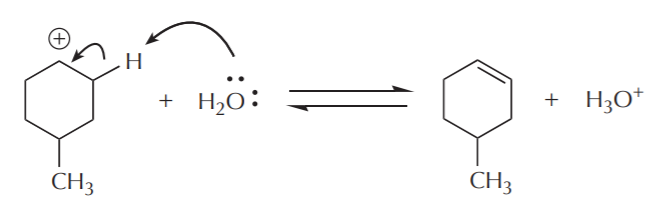
Notice that each step in this mechanism is reversible. This means that the acid-catalyzed alcohol dehydration is an equilibrium process. As such, equilibrium must be manipulated to shift the outcome towards the desired product. This reaction enables us to make alkenes from alcohols, or alcohols from alkenes. Typically, formation of alkenes is favored by use of concentrated acid, whereas formation of alcohols is favored by use of dilute acid. The following sequence shows the steps in reverse, starting with an alcohol, and arriving at an alkene.