15.1: Synthetic Drawbacks of Sn1 Reactions
- Page ID
- 216042
In terms of synthetic value, any reactions whose mechanism involves carbocation formation suffer from some drawbacks. Once formed, carbocations can undergo several process that may result in formation of undesired side products. In the context of Sn1 reactions, some of the things that carbocations can do are:
1) They can go on to form the expected Sn1 products.
2) They can rearrange to form products whose connectivities are changed relative to that of the original substrate.
3) They can undergo elimination (E!) reactions to form alkenes
Examples:
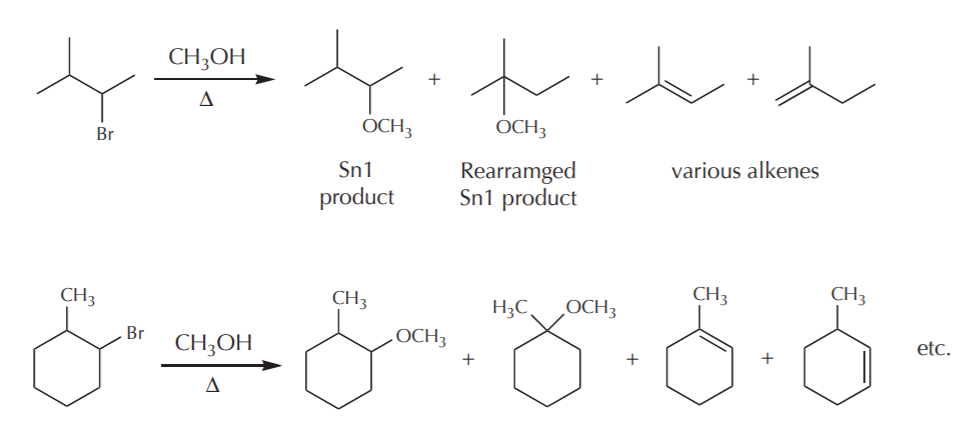
This limits the synthetic usefulness of such reactions, for one has to deal with mixtures of products and the separation of the desired ones.
In fact, Sn1 and E1 reactions typically go hand in hand and are difficult to disassociate, because they share similar characteristics, and the conditions that favor one also favor the other. We’ve already learned the characteristics of Sn1 reactions and the factors that favor them. We can extend that to E1 reactions as well:
Characteristics of the Sn1 and E1 mechanisms:
a) They are multistep processes
b) They occur with formation of carbocation intermediates in the rate determining step
c) They follow first order (unimolecular) kinetics. That is, rate=k[substrate]
Sn1 and E1 mechanisms are favored by using:
a) Sterically hindered substrates
b) Weak (neutral), small nucleophiles and heating
c) Moderate to high polarity solvents
The Sn1 mechanism leads to substitution products, and the E1 mechanism leads to formation of alkenes.
The same substrates that are prone to undergo Sn1 reactions also undergo E1 reactions. They are of two major types:
a) Secondary and tertiary alky halides
b) Secondary and tertiary alcohols.


