14.3: Factors That Affect The Course of Nucleophilic Substitutions at sp3 Carbon
- Page ID
- 216036
1. STERIC NATURE OF THE SUBSTRATE. Steric accessibility of the electrophilic center in the substrate is probably the most important factor that determines if a nucleophilic substitution will follow an Sn1 or an Sn2 mechanism.
EXAMPLES OF Sn2 (sterically accessible) SUBSTRATES
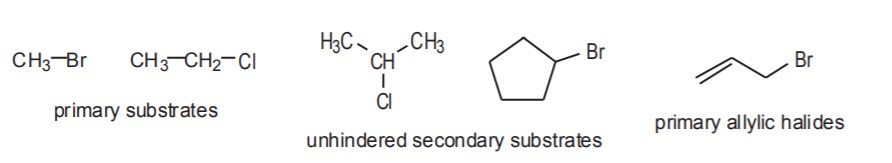
EXAMPLES OF Sn1 (sterically hindered) SUBSTRATES
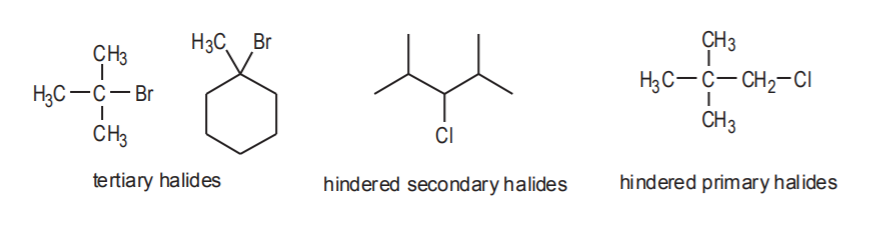
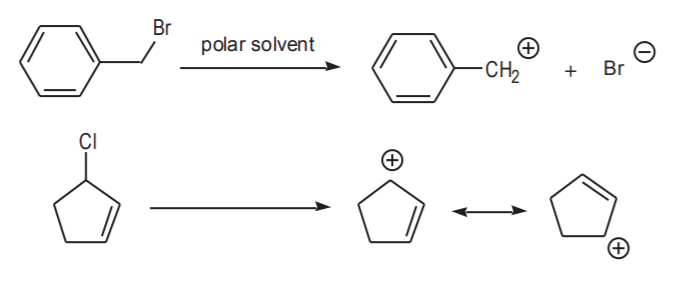
Some substrates, whether they are sterically hindered or not, may prefer to undergo Sn1 reactions if they can dissociate into very stable carbocations in the presence of the solvent. In most cases this involves resonance-stabilized cations.
EXAMPLES OF Sn1 SUBSTRATES THAT FORM STABLE CARBOCATIONS
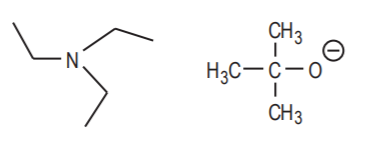
2. NATURE OF THE NUCLEOPHILE. Both Sn1 and Sn2 reactions prefer small nucleophiles. Large nucleophiles have more difficulty accessing the electrophilic center in the substrate. They also have increased tendency to act as Bronsted bases, seeking acidic protons rather than electrophilic centers due to the lower activation energy of acid-base reactions compared to nucleophilic substitutions.

Weak, small nucleophiles that favor Sn1 reactions are shown below. Notice that several are the conjugate acids of strong nucleophiles. They are also typically neutral, but some have a delocalized negative charge.

Large nucleophiles, especially if they are strong, have a tendency to act as Bronsted bases rather than as nucleophiles. They should be avoided if a nucleophilic reaction is desired. Examples are:
3. SOLVENT USED. It has already been mentioned that Sn2 mechanisms are favored by low to moderate polarity solvents such as acetone and N,N-dimethylformamide (DMF). Sn1 mechanisms are favored by moderate to high polarity solvents such as water and alcohols. It is frequently the case that in Sn1 reactions the solvent also doubles as the nucleophile. Water and alcohols are prime examples of this practice.
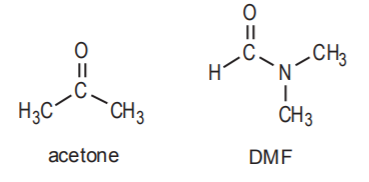
Sn2 Solvents
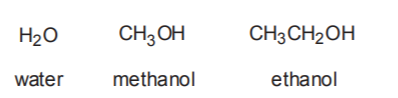
Sn1 Solvents
4. LEAVING GROUP. The nature of the leaving group has more of an effect on the reaction rate (faster or slower) than it does on whether the reaction will follow an Sn1 or an Sn2 mechanism. The most important thing to remember in this regard is that good leaving groups are weak bases.
a) All halogens, except for fluorine, are good leaving groups
b) Groups that leave as resonance stabilized ions are also weak bases and therefore good leaving groups.
c) Water is a good leaving group frequently used to prepare alkyl chlorides and bromides from alcohols.
The OH group in alcohols is not a good leaving group because it leaves as hydroxide ion, which is a strong base. However, if the hydroxyl group is protonated first with strong acid, it can leave as a water molecule, which is a good leaving group. Refer to the manuscript titled Introduction to Lewis Acid-Base Chemistry for a discussion and examples of this approach.


