14.1: Sn2 Reactions
- Page ID
- 216027
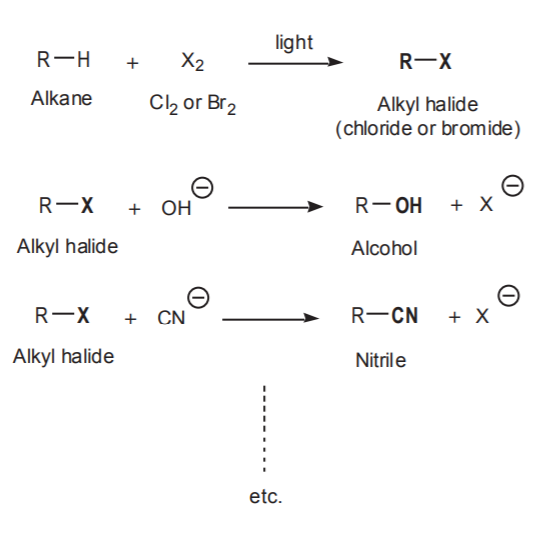
From a synthetic point of view, this is the most useful reaction. It provides a means to prepare many functional groups from alkyl halides, and therefore from alkanes through the free radical halogenation reaction.
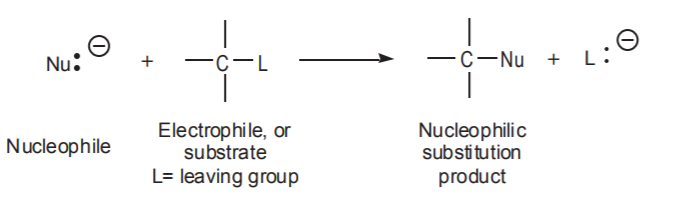
The Sn2 mechanism: a) is a single step process b) involves no intermediates c) involves only one transition state, which is of low polarity d) follows second order (bimolecular) kinetics. That is, rate=k[substrate][nucleophile]
In nucleophilic substitutions at sp3 carbon, Sn2 mechanisms are favored by using: a) sterically accessible substrates b) strong (negatively charged), small nucleophiles c) low to moderate polarity solvents.
Stereochemically, if the electrophilic center in the substrate is chiral, the Sn2 reaction produces a product with inverted configuration.


