12.3: Recognizing Electrophiles
- Page ID
- 216022
There are two requirements for a molecule to be considered a good electrophile. First, it must contain an electrophilic center or atom. Second, the electrophilic atom must be able to accommodate a new sigma bond. Please keep in mind the difference between electrophile and electrophilic center. The term electrophile refers to the molecule. The term electrophilic center refers to the particular part of the molecule susceptible to nucleophilic attack.
To avoid confusion, the term substrate is frequently used in reference to electrophiles. This term denotes a molecule being acted upon by another agent. For example, an enzyme substrate is a molecule being modified by an enzyme. Likewise, an electrophile can be thought of as the substrate of a nucleophile when the latter “attacks” its electrophilic center.
Electrophilic centers are areas of low electron density. Most often they are atoms which (a) contain an incomplete octet, and/or (b) carry a full or a partial positive charge. A partial positive charge can be revealed by writing resonance structures, or by identifying a polar bond.
1. The following are examples of electrophiles containing atoms with incomplete octets:
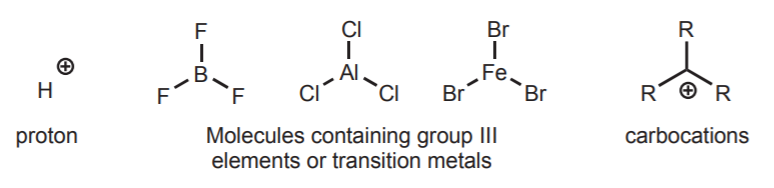
2.These are examples of electrophiles containing atoms with partial positive charges:
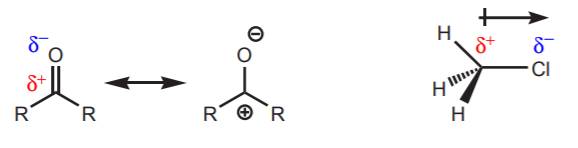
3. Atoms of the second row like oxygen and nitrogen, which are more electronegative than carbon, seldom act as electrophilic centers, even if they carry a positive charge. In that situation they seek to lessen their positive character by sharing the charge with adjacent atoms, causing them go become acidic (protons) or electrophilic (carbon for instance). Resonance structures can reveal this shift of positive charge.
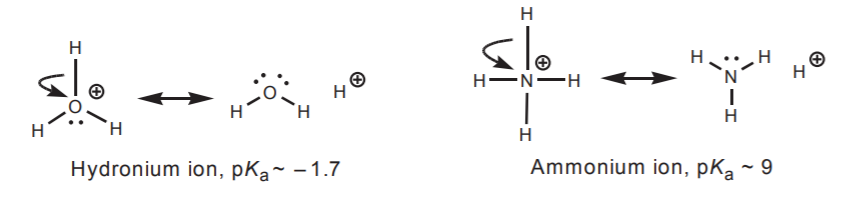
The ammonium and hydronium ions are of moderate to high acidity because the highly electronegative oxygen and nitrogen seek to transfer their positive charge to the adjacent proton, making it acidic. In addition, those atoms cannot accomodate another bond without violating the octet rule.

In addition to making the protons acidic, a positive charge on oxygen can also make the adjacent carbon electrophilic by a similar transfer of positive character. Moreover, the oxygen atom becomes part of a good leaving group, in this case water. The stage is set for either a nucleophilic attack on carbon, or a reaction with a Bronsted base.
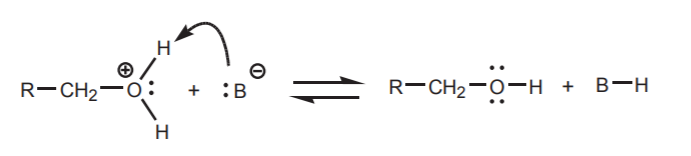
The reaction with a base (B - ) is an equilibrium process that normally has a low activation energy and is therefore relatively fast.
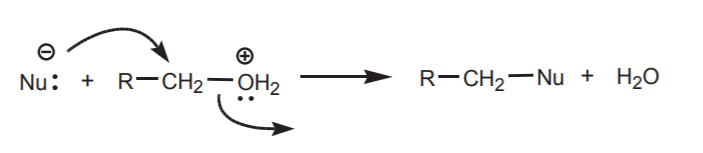
The reaction with a nucleophile (Nu - ) is a kinetic process that normally has a higher activation energy than a proton transfer and is therefore slower. If the nucleophile being used is also a good base, it will prefer to take the proton.
An example of the above is the reaction of protonated n-butanol with either bromide ion or ammonia. Bromide ion is one of the best nucleophiles, but a weak base. It prefers to act as a nucleophile. Ammonia is a moderately good nucleophile but also a good base. Given the choice, it prefers to act as a base.
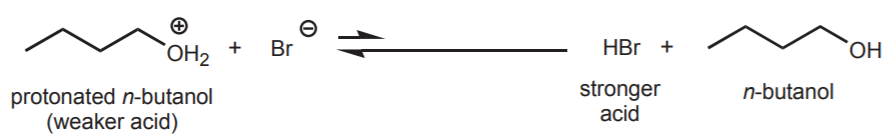
acid-base equilibrium favors the left side because bromide ion is a weak base
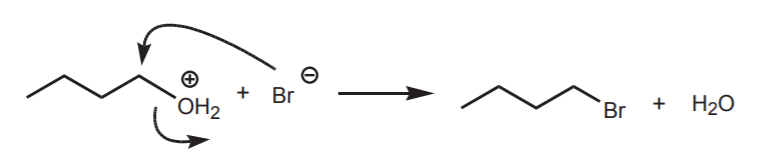
Bromide ion is an effective nucleophile, preferring to attack the electrophilic carbon displacing the water

Ammonia is a weak nucleophile and a moderate base. As long as it can act as a base, it will prefer to do so rather than engaging in a higher energy nucleophilic displacement.
4. Unlike their second row counterparts, some electronegative elements of the third row such as sulfur and phosphorus can sometimes act as electrophilic centers due to their larger size and the ability to accommodate a new sigma bond using their d-orbitals:
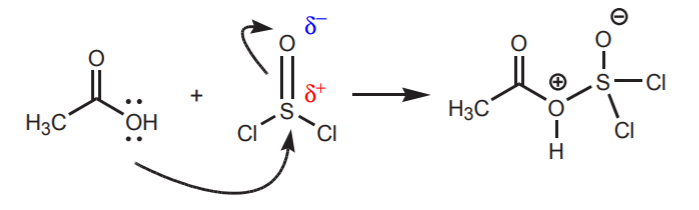
Unlike oxygen, sulfur can be electrophilic because it can accommodate a new sigma bond using its d-orbitals without violating the octet rule.


