11.13: Identifying Bronsted Bases
- Page ID
- 215878
Basic sites in organic molecules are areas of high electron density. We’ve already talked about π-bonds as examples of such areas. However, the large majority of Bronsted bases comprises molecules that have atoms with nonbonding electrons (lone pairs). The following trends can be observed.
1. Nonbonding electrons are the most readily available for reaction with acids because no energy has to be invested in breaking a bond before they can be used. The strongest Bronsted bases contain atoms with unshared electrons which are localized. Examples are:
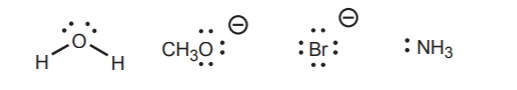
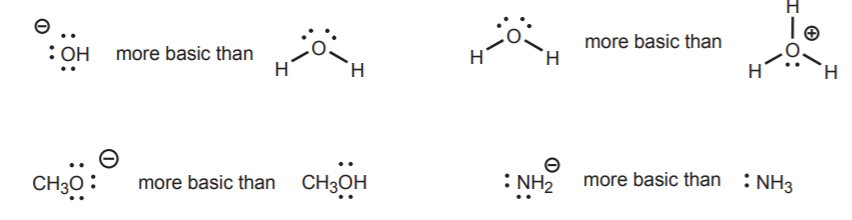
2. In a conjugate acid-base pair, the more negatively charged (or less positively charged) partner is the most basic. Examples of relative basicities in such pairs are:
3. The periodic trend in basicity of atoms is the reverse of the acidity trend. This is particularly useful when comparing conjugate bases. Other factors being comparable, the basicity of the atom increases from bottom to top and from right to left. Examples are:
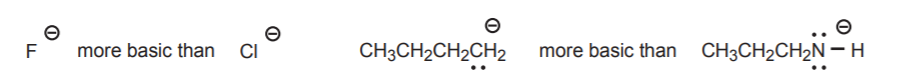
4. The above can also be applied to resonance structures. This concept can be used to predict the outcome of an acid-base reaction when there are several possible reactive sites.

5. Electron delocalization decreases the basic character of the atom. Delocalization stabilizes the molecule and makes it less reactive. Delocalized electrons are not as available as localized electrons because they are diffused over a larger area.

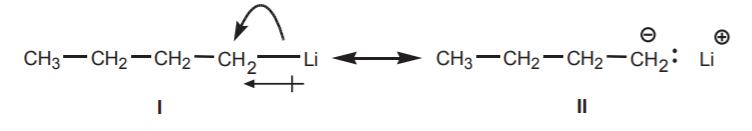
6. Resonance structures involving π-bonds are the most commonly seen type. However, resonance structures involving σ-bonds can be written when the bond is highly polarized, as in the case of a C-Li bond. Such structures often reveal reactivity sites not obvious from the main contributor. In the example below, structure II reveals the basic character of the carbon atom.
7. Alkenes are considered weak bases, since the π-bond must be broken before its π-electrons can be used for reaction with an acid.
8. Finally, a pKa table can provide the best measure of basicity when properly used.


