11.12: Functional Groups and Reactivity Sites in Organic Molecules
- Page ID
- 215877
As explained before in relation to functional groups, the very definition of this term is as a reactive center, or site, in the molecule. We can view an organic molecule as consisting of two major structural categories: the basic carbon skeleton, and functional groups.
The basic carbon skeleton is an alkane-like structure, made up of only sp3 carbons and hydrogen atoms, and therefore only sigma (single) bonds. It constitutes the framework that supports the reactive sites, or functional groups. The carbon skeleton is for most practical purposes considered a nonpolar part of the molecule. It is also considered to be nonreactive, except under very harsh, extreme, or special conditions.
Alkanes contain no functional groups, but as soon as their structure is chemically modified in any way that leads to relatively stable products, functional groups are introduced. Some of the simplest modifications that introduce functional groups are the creation of a π-bond and the introduction of a heteroatom. Technically speaking, heteroatoms are atoms other than carbon and hydrogen. The term is most commonly used in reference to nonmetals of the second and third rows. That is, N, O, S, P, and the halogens.
The introduction of π-bonds, as in alkenes and alkynes, does not affect the polarity of the molecule very much, but it affects the flexibility of it by restricting free rotation around carbon-carbon bonds. The presence of a π-bond introduces a center of reactivity by increasing the electron density at that site. At the same time, “mobile,” or “available” electrons are introduced. Remember that π-bonds are weaker than σ-bonds, and therefore π-electrons are easier to move for use in chemical reactions.
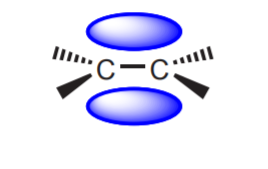
The π-bond is a high electron density region. π-electrons can be used for chemical reactions under the right conditions
The introduction of heteroatoms such as oxygen almost invariably introduces a dipole, since most of these elements are more electronegative than carbon and hydrogen. By introducing a dipole, an electron imbalance is created that results in formation of a low electron density area and a high electron density area. The low electron density area will then be reactive towards electron-rich molecules. The high electron density area will be reactive towards electron-poor (deficient) molecules.
Identifying reactive sites in organic molecules amounts to identifying areas of low or high electron density. In the case of carboncarbon double or triple bonds (alkenes and alkynes), the region is an area of high electron density. Such molecules typically act as electron donors, even though the π-bond must be broken before those electrons become available. If we’re not dealing with π-bonds, then we must identify the strongest dipoles in the molecule and the areas of low or high electron density they create, such as in the examples below.

Obviously, when it is a hydrogen atom that is positioned at the δ+ end of the dipole, we have an acidic proton that can engage in reactions with Bronsted bases. We have already learned to identify acidic protons, but how do we identify basic sites in molecules?


