11.7: Acid-Base Reactions as "Proton" Transfers
- Page ID
- 215762
When a Bronsted acid (or simply acid) reacts with a Bronsted base (or simply base) a proton is transferred from the acid to the base. This results in formation of another acid, called the conjugate acid, and another base, called the conjugate base. For example, when hydroxide ion (a base) reacts with hydrogen chloride (an acid), a new acid (water) is formed. Water is then the conjugate acid of hydroxide ion. Likewise, a new base (chloride ion) is formed. Chloride ion is then the conjugate base of hydrogen chloride. The reaction is an equilibrium process because the new acid and the new base can react together to revert to the original reactants. Therefore we can also say that hydroxide ion is the conjugate base of water, and that hydrogen chloride is the conjugate acid of chloride ion. This relativity of concepts is characteristic of the Bronsted-Lowry acid-base theory.

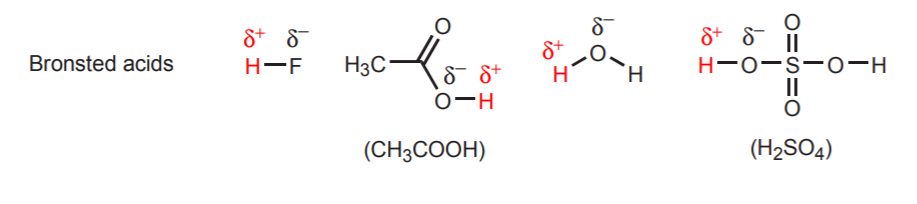
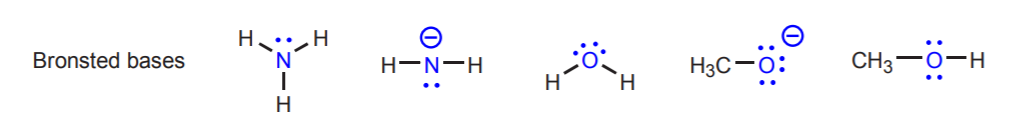
Conjugate acid-base pairs differ only by a proton. Other examples of conjugate acid-base pairs are H2O / H3O+ and NH3 / NH4+. The above reaction also shows the direction of electron movement. In acid-base reactions, electron movement always originates at the base and moves towards the acidic proton in the acid. The base is the electron-rich species. We can identify bases because they usually have an atom with unshared electron pairs (lone pairs). Sometimes this atom also carries a negative charge, but this is not a requirement. Likewise, we can identify the acid because it is the molecule that has acidic protons (hydrogens that carry a strong partial positive charge). The following are examples of other acids and bases. The acidic protons are shown in red, and the basic atoms in blue. Keep in mind that the concept of acid or base is always relative in the Bronsted theory. Some molecules such as water can act as acids or as bases, depending on who they are reacting with. We will expand on this later.