10.4: Bond Breaking and Bond Formation
- Page ID
- 215752
The simplest and first process that takes place during a chemical transformation is the breaking of a bond. The following discussion is confined to covalent bonds because they are the most prevalent in organic molecules. Breaking bonds always requires an energy input. A free energy diagram for the breaking of a covalent bond between atoms A and B would have the following appearance.
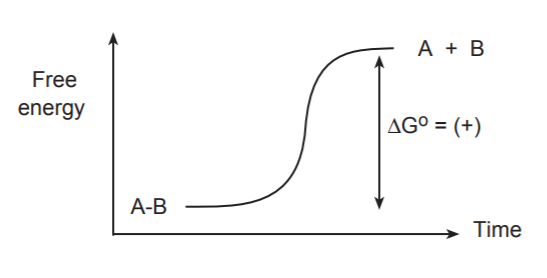
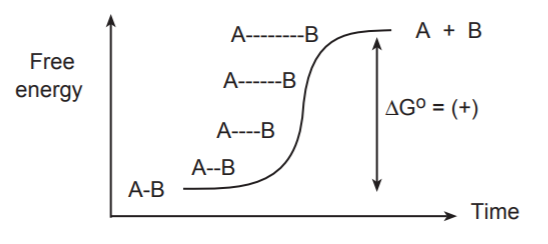
If we were to visualize the breaking of this bond “frame by frame,” we would see the bond stretching gradually until it snapped. At the same time we would see a gradual increase of the free energy of the system.
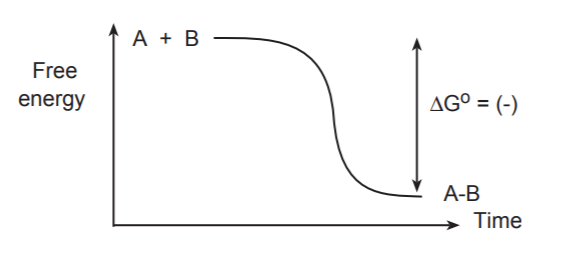
The process of bond formation, being the opposite of bond breaking, always releases energy. That is, lowers the energy of the system.
NOTE: This discussion assumes that the reader is familiar with basic thermodynamic concepts such as free energy, enthalpy, entropy, etc. Refer to the appropriate sections in your textbook for detailed explanations (Sections 4-4 and 4-5 in the Wade textbook, 4th and 5th editions)


