9.3: Shortcuts for Assigning Absolute Configuration on Paper
- Page ID
- 215737
According to the Cahn-Ingold-Prelog convention, when assigning absolute configuration to a chiral carbon the lowest priority group that’s attached to that carbon must be pointing away from an observer who is looking at the carbon in question. On paper, that usually means that if the observer is the person looking at the page, then the lowest priority group is pointing away from the observer, going behind the plane of the paper. In a 3-D formula this is indicated thus:
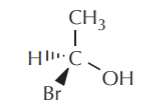
When the formula is given to us in this way, it’s easy to assign configuration. All we have to do is assign priorities to the other three substituents and see if they are arranged clockwise or counterclockwise when the observer follows them in order of decreasing priorities. We don’t’ have to mentally reposition either ourselves or the molecule in any way. In the example given above we can see that the central carbon has the (S) configuration.

If the lowest priority group is not presented to us already positioned towards the back of the chiral carbon, then it is useful to remember the following basic principle:
Every time any two substituents are exchanged, the opposite configuration results
With this in mind, we can encounter two possible scenarios: Either the lowest priority group is positioned in front of the chiral carbon, or on the plane of the paper. If the lowest priority group is positioned in front of the chiral carbon (that is, opposite where it should be, according to the rules) we can still assign configuration by following the arrangement of the other three groups as given to us, but the configuration we obtain will be the opposite of the actual one. Following the same example given above we have:
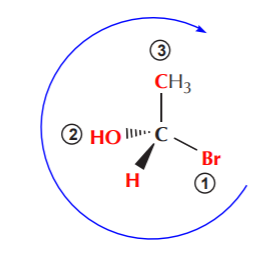
With the lowest priority group positioned in the front rather than towards the back, the central carbon appears to have the (R) configuration. The actual configuration is therefore (S). This is best seen when we rotate the molecule until the H atom is in the correct orientation.
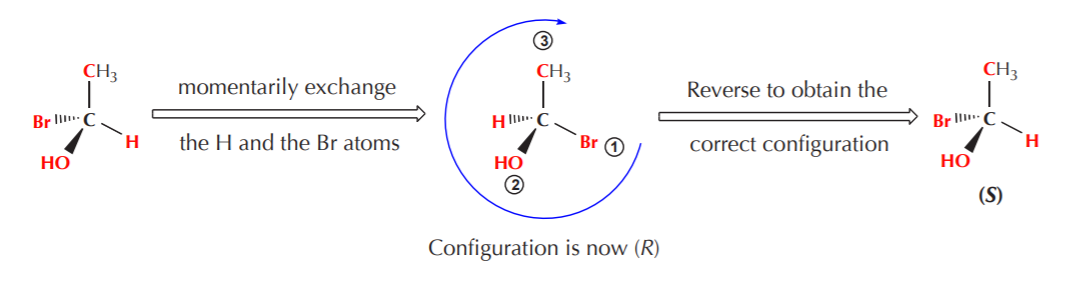
If the lowest priority group is positioned on the plane of the paper, we can momentarily exchange it with whatever group happens to be positioned in the back, then assign configuration, then reverse it.
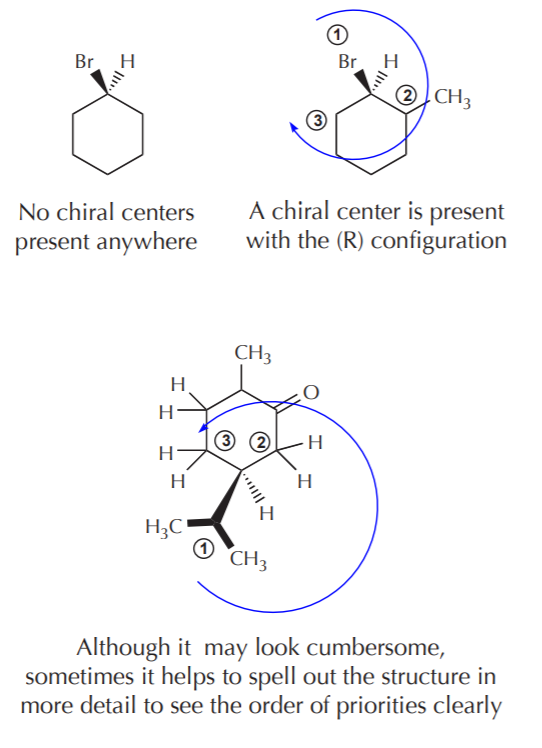
ASSIGNING ABSOLUTE CONFIGURATIONS IN CYCLIC MOLECULES
Cyclic molecules are frequently represented on paper in such a way that the ring atoms are all lying on the plane of the paper, and substituents are either coming out of the paper towards the front or towards the back. It is therefore easy to assign configuration to any chiral centers forming part of the ring, since the lowest priority substituent will be either pointing to the front or to the back. However, always make sure there is in fact a chiral center present. The fact that a 3-D representation is given does not necessarily mean there is a chiral center in the molecule.
ASSIGNING ABSOLUTE CONFIGURATIONS IN FISCHER FORMULAS
The key points to keep in mind regarding Fischer projection formulas are:
1. Horizontal lines represent bonds to the chiral carbon that are coming out of the plane of the paper towards the front, whereas vertical lines represent bonds going behind the plane of the paper towards the back. Thus, Fischer formulas are easily translated into “bow tie” formulas, which are 3-D formulas.

2. The lowest priority group bonded to the chiral carbon must always be shown as a horizontal bond.
The process of assigning (R) or (S) configuration to the chiral carbon is the same as outlined before, but since the lowest priority group is pointing towards the front, the configuration obtained directly from a Fischer formula is the opposite of the actual one.
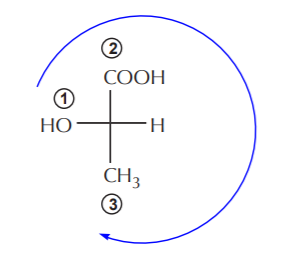
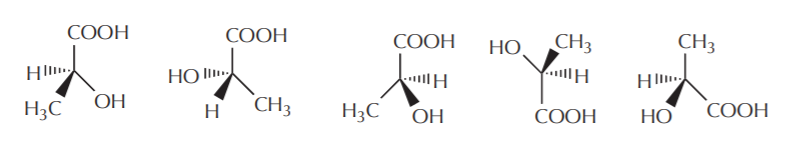
The order of priorities follows a clockwise direction in the Fischer formula. Therefore the actual configuration of this molecule is (S).
Once we know the actual configuration, we can represent the molecule in any of several possible ways using 3-D formulas. Thus the formulas shown below all represent the same molecule as given above in Fischer projection form. That is to say, all have the (S) configuration at the central carbon.