7.4: Haloalkanes, or Alkyl Halides
- Page ID
- 215730
Like alkanes, alkyl halides, called haloalkanes by IUPAC rules, can have common names as well as systematic names. Common names are widely used because they are short and easy to say and write. Once you know what chloroform is, it’s easier to refer to it as such, rather than as trichloromethane.
COMMON NAMES -
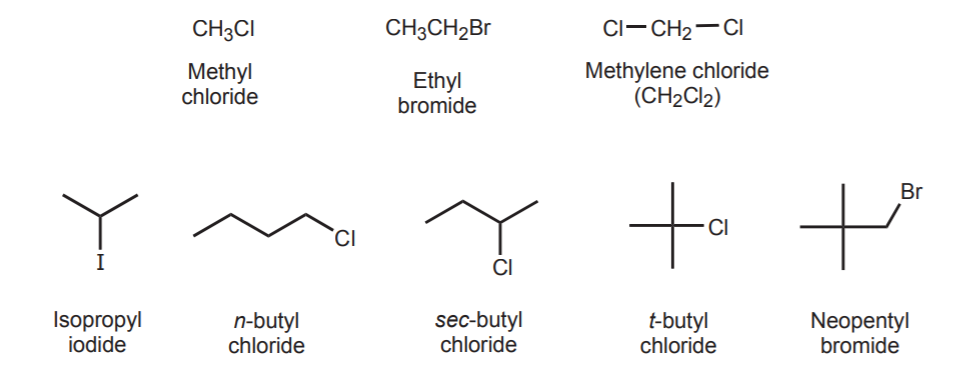
Most common names refer to the main carbon frame as an alkyl group, and then add the halogen name, ending in -ide. Names such as isopropyl, or sec-butyl are extensively used. Likewise, primary halides have the halogen attached to a primary carbon, secondary halides have the halogen attached to a secondary carbon, etc. Refer to chapter 6 in your textbook. Examples are:
IUPAC NAMES -
The systematic naming of haloalkanes follows the same basic principles as that of alkanes. Find the longest carbon chain and number it trying to obtain the lowest set of numbers. Follow the alphabetical rule in naming the substituents, whether they are alkyl groups or halogens. Use prefixes such as di-, tri, as needed. The following examples illustrate these points.
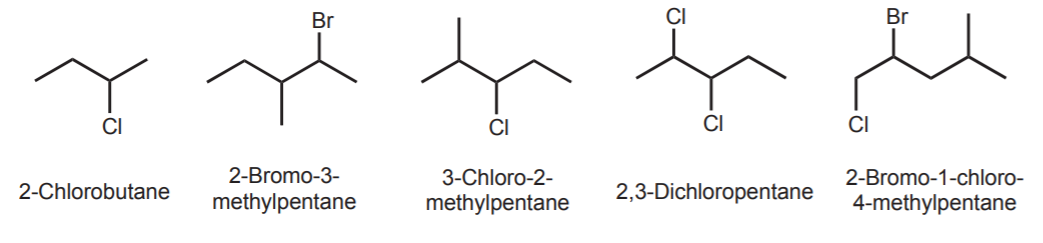
If two substituents are equally far from either end, number the chain according to alphabetical order.

Name complex substituents using parenthesis and the guidelines discussed before for alkanes.
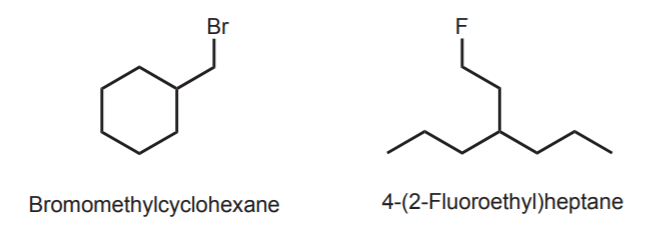
All these rules apply only if the substituents on the main carbon chain are alkyl groups and/or halogens. Other functional groups have different priorities, depending on their nature.
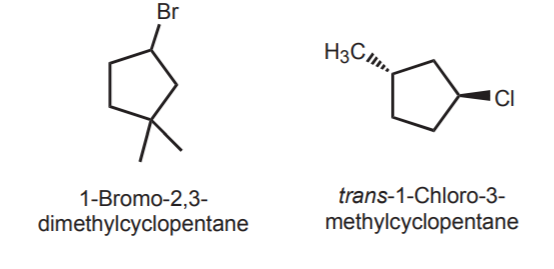
To name cyclohaloalkanes that contain halogen and alky substituents, number from the carbon bearing the halogen. Remember to indicate cis or trans when applicable.