6.1: Mobility of Pi Electrons and Unshared Electron Pairs
- Page ID
- 215683
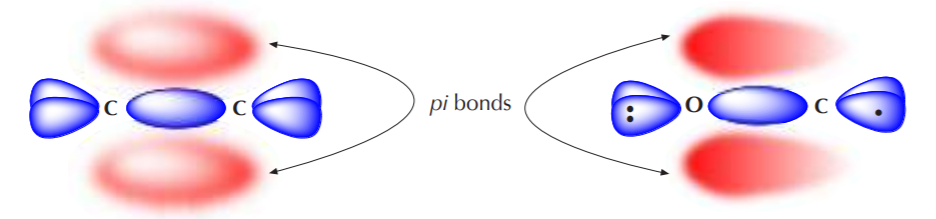
Now that we understand the difference between sigma and pi electrons, we remember that the pi bond is made up of loosely held electrons that form a diffuse cloud which can be easily distorted. This can be illustrated by comparing two types of double bonds, one polar and one nonpolar. The C=C double bond on the left below is nonpolar. Therefore the pi electrons occupy a relatively symmetric molecular orbital that’s evenly distributed (shared) over the two carbon atoms. The C=O double bond, on the other hand, is polar due to the higher electronegativity of oxygen. The pi cloud is distorted in a way that results in higher electron density around oxygen compared to carbon. Both atoms still share electrons, but the electrons spend more time around oxygen. The drawing on the right tries to illustrate that concept.
Using simple Lewis formulas, or even line-angle formulas, we can also draw some representations of the two cases above, as follows.
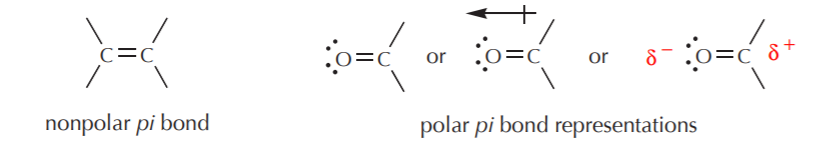
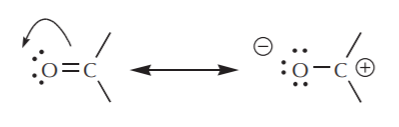
The dynamic nature of pi electrons can be further illustrated with the use of arrows, as indicated below for the polar C=O bond:
The CURVED ARROW FORMALISM is a convention used to represent the movement of electrons in molecules and reactions according to certain rules. We’ll study those rules in some detail. For now, we keep a few things in mind:
a) Curved arrows always represent the movement of electrons, not atoms.
b) Electrons always move towards more electronegative atoms or towards positive charges.
We notice that the two structures shown above as a result of “pushing electrons” towards the oxygen are RESONANCE STRUCTURES. That is to say, they are both valid Lewis representations of the same species. The actual species is therefore a hybrid of the two structures. We conclude that:
Curved arrows can be used to arrive from one resonance structure to another by following certain rules.
Just like pi electrons have a certain degree of mobility due to the diffuse nature of pi molecular orbitals, unshared electron pairs can also be moved with relative ease because they are not engaged in bonding. No bonds have to be broken to move those electrons. As a result, we keep in mind the following principle:
Curved arrows usually originate with pi electrons or unshared electron pairs, and point towards more electronegative atoms, or towards partial or full positive charges.
Going back to the two resonance structures shown before, we can use the curved arrow formalism either to arrive from structure I to structure II, or vice versa.
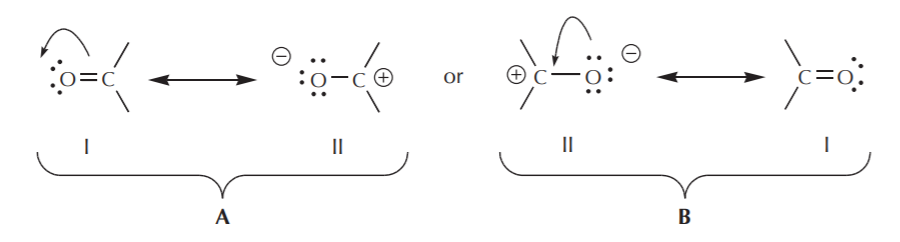
In case A, the arrow originates with pi electrons, which move towards the more electronegative oxygen. In case B, the arrow originates with one of the unshared electron pairs, which moves towards the positive charge on carbon. We further notice that pi electrons from one structure can become unshared electrons in another, and vice versa. We’ll look at additional guidelines for how to use mobile electrons later.
Finally, in addition to the above, we notice that the oxygen atom, for example, is sp2 hybridized (trigonal planar) in structure I, but sp3 hybridized (tetrahedral) in structure II. So, which one is it? Again, what we are talking about is the real species. The real species is a hybrid that contains contributions from both resonance structures. In this particular case, the best we can do for now is issue a qualitative statement: since structure I is the major contributor to the hybrid, we can say that the oxygen atom in the actual species is mostly trigonal planar because it has greater sp2 character, but it still has some tetrahedral character due to the minor contribution from structure II. We’ll explore and expand on this concept in a variety of contexts throughout the course.
What about sigma electrons, that is to say those forming part of single bonds? These bonds represent the “glue” that holds the atoms together and are a lot more difficult to disrupt. As a result, they are not as mobile as pi electrons or unshared electrons, and are therefore rarely moved. There are however some exceptions, notably with highly polar bonds, such as in the case of HCl illustrated below. We will not encounter such situations very frequently.
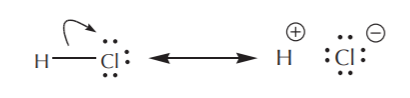
This representation better conveys the idea that the H–Cl bond is highly polar.


