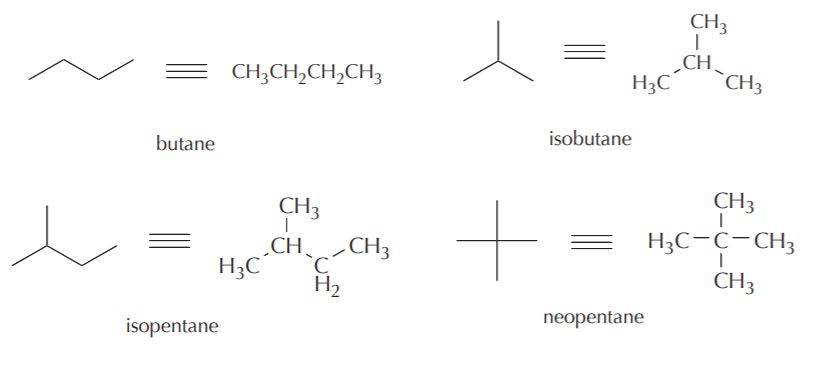
5.3: Sigma Bonding
- Page ID
- 215676
When atomic orbitals (pure or hybrid) of different atoms overlap to form covalent bonds, they may approach each other in two major ways: head to head, or sideways. Only head to head overlap is possible with s orbitals because they are spherical. Hybrid orbitals also undergo mostly head to head overlap when forming covalent bonds. p-orbitals, on the other hand, can approach each other either sideways or head to head. For now, however, we are concerned only with head to head overlap because that’s the only type that occurs in alkanes. We’ll discuss sideways overlap later in connection with alkenes and alkynes, that is, hydrocarbons that have double and triple bonds respectively.
When orbitals approach each other in a head to head fashion, the resulting covalent bonds are called sigma bonds.
As illustrations, consider the bonds that have already been studied. The bond between two hydrogen atoms is an example of sigma bonding. The bonds between the sp3 orbitals of hybridized carbon and the s orbitals of hydrogen in methane are also example of sigma bonds.
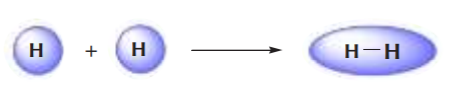 sigma bond between s orbitals
sigma bond between s orbitals
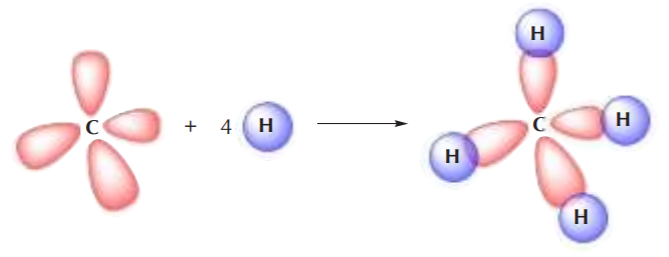 sigma bonds between s and sp3 orbitals
sigma bonds between s and sp3 orbitals
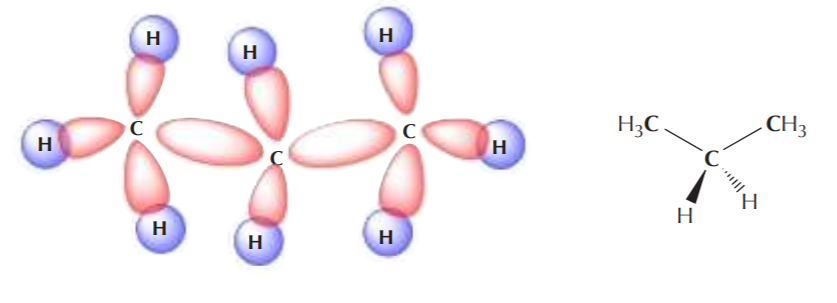
Two sp3 carbons can also overlap to form a C–C sigma bond where two sp3 orbitals overlap head to head, such as in the formation of the ethane molecule:
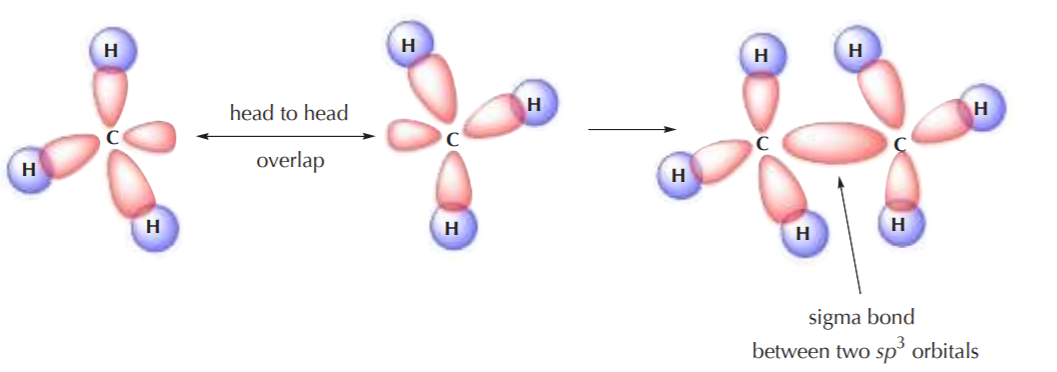
It can be easily seen that the only type of covalent bonds present in alkanes are sigma bonds, also loosely known as single bonds.
LINE-ANGLE FORMULAS
In alkanes of 3 carbon atoms or more, the main carbon chain acquires a zig-zag structure due to the 109.5o angle between C–C bonds, such as in propane:
Two representations of propane, where the zig-zag structure of the carbon chain becomes apparent
Writing Lewis formulas, or even condensed formulas, for alkanes of many carbon atoms can quickly become cumbersome. A short hand notation that uses zig-zag lines has been developed. The resulting representations are known as line-angle formulas. The beginning and the end of the zig-zag line, as well as any breaks in direction represent carbon atoms.
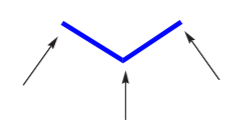
Line angle representation for propane equivalent to 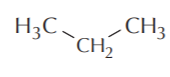 or CH3CH2CH3 The arrows point to the positions of the carbon atoms.
or CH3CH2CH3 The arrows point to the positions of the carbon atoms.
Every carbon atom has to form 4 bonds. The bonds that are not shown are assumed to be bonds to hydrogen. Other examples are: