3.1: Learning Objectives, Valence Electrons, Octet Rule
- Page ID
- 214216
LEARNING OBJECTIVES
To introduce the basic principles of covalent bonding, different types of molecular representations, bond polarity and its role in electronic density distributions, and physical properties of molecules.
VALENCE ELECTRONS
They are those found in the highest energy level of the atom, or outer shell. In the periodic table, the number of valence electrons is given by the group number. For example, in the second row, the nonmetals are:

| BORON | Group III | 3 valence electrons | 2s2, 2p1 |
| CARBON | Group IV | 4 valence electrons | 2s2, 2p2 |
| NITROGEN | Group V | 5 valence electrons | 2s2, 2p3 |
| OXYGEN | Group VI | 6 valence electrons | 2s2, 2p4 |
| FLUORINE | Group VII | 7 valence electrons | 2s2, 2p5 |
OCTET RULE
The atoms that participate in covalent bonding share electrons in a way that enables them to acquire a stable electronic configuration, or full valence shell. This means that they want to acquire the electronic configuration of the noble gas of their row. Obviously the name of this rule is a misnomer. Helium, the noble gas of the first row, has only two electrons. Hydrogen, the only element in the first row besides Helium, fulfills the “octet rule” by sharing two electrons only.
ELECTRON SHARING IN THE HYDROGEN MOLECULE
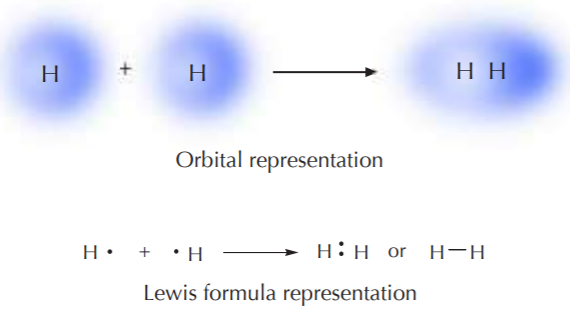
Two hydrogen atoms form a covalent bond to make a hydrogen molecule. Each contributes one electron and forms a system that is much more stable than the isolated atoms. Although the orbital representation is more visually telling, the Lewis formula representation is easier to write, and therefore will be used from now on, unless there is reason to do otherwise.
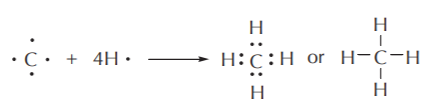
The elements of the second row fulfill the octet rule by sharing eight electrons, thus acquiring the electronic configuration of neon, the noble gas of this row. Besides hydrogen, most of the elements of interest in this course are the second row nonmetals: C, N, O, and the halogens. As the building block of all organic molecules, carbon is of particular interest to us. Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons, while each hydrogen shares 2. Thus every atom in this stable molecule fulfills the octet rule.
ELECTRON SHARING IN THE METHANE (CH4) MOLECULE