2.1: Learning Objectives and Basic Concepts
- Page ID
- 214179
LEARNING OBJECTIVES
To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential to the understanding of organic molecular structure and, later on, reaction mechanisms.
BASIC CONCEPTS
Most of this material is a review of general chemistry. You might find it helpful to keep a general chemistry textbook available for reference purposes throughout the organic chemistry course.
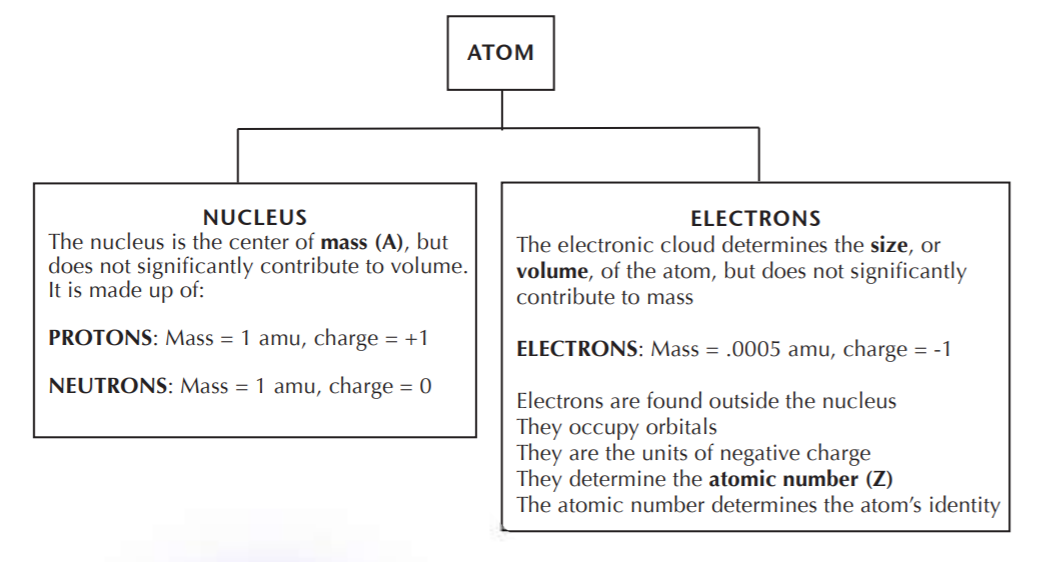
The following diagram summarizes the basic facts of the structure of the atom.
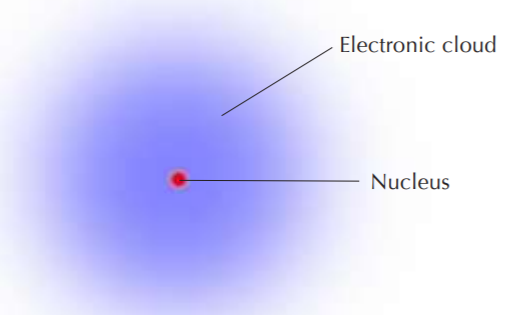
A simplified view of the hydrogen atom, which consists of only one electron outside the nucleus. The nucleus contains only one proton and no neutrons. All other elements contain neutrons in their nuclei.
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic number (Z) at the lower corner.
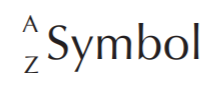
Examples: 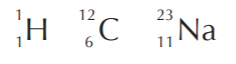
Electron trade constitutes the currency of chemical reactions. The number of electrons in a neutral atom (that is, the atomic number) gives the element its unique identity. No two different elements can have the same atomic number. The periodic table is arranged by order of increasing atomic number, which is always an integer.
In contrast to the atomic number, different forms of the same element can have different masses. They are called isotopes. The following are representations for some of the isotopes of hydrogen and carbon.

The atomic mass reported in the periodic table for any given element is actually a weighted average of the masses of its isotopes as found in nature. Thus the mass of carbon is reported as 12.01115 rather than 12.00000 because it contains the relative contributions of both isotopes. The natural abundance of carbon12 is nearly 100%, whereas that of carbon-13 is only about 1%. The reported mass is slightly greater than 12.00000 because of the small contribution of carbon-13. Therefore the mass number, as found in periodic tables, does not have to be an integer like the atomic number.


