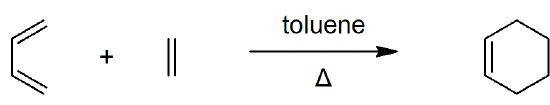14.S: Conjugated Compounds and Ultraviolet Spectroscopy (Summary)
- Page ID
- 212875
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Concepts & Vocabulary
- Dienes that consist of two double bonds separated by a single bond are conjugated.
- Dienes that have the double bonds separated by more than one single bond are isolated (non-conjugated)
14.1 Stability of Conjugated Dienes - Molecular Orbital Theory
- Conjugated dienes are more stable than non-conjugated dienes.
- Electrons in conjugated dienes are delocalized due to overlap of all 4 p-orbitals.
- Molecular orbitals show stability of dienes and allylic carbocations.
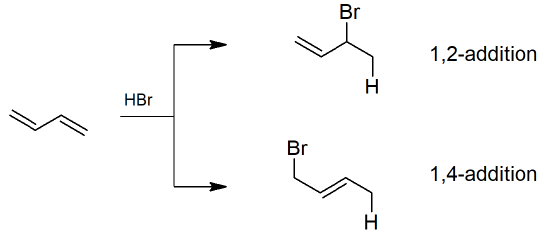
14.2 Electrophilic Additions to Conjugated Dienes - Allylic Carbocations
- Addition to conjugated dienes occur at multiple positions due to resonance of the allyl carbocation intermediate called 1,2 and 1,4 addition.
14.3 Kinetic vs. Thermodynamic Control of Reactions
- Lower transition states lead to kinetic products.
- More stable products lead to thermodynamic products.
- Kinetic reaction conditions favor 1,2 addition to conjugated dienes.
- Thermodynamic reaction conditions favor 1,4 addition to conjugated dienes.
- For some reactions, the kinetic and thermodynamic products are the same molecule.
14.4 The Diels-Alder Cycloaddition Reaction
- Cycloaddition reactions involve concerted bonding of two independent pi-electron systems for form a new ring.
- The Diels-Alder reaction is a widely used [4+2] cycloaddition that froms two new sigma bonds.
14.5 Characteristics of the Diels-Alder Reaction
- The Diels-Alder reaction is stereospecific with cis dienophiles yielding cis substitution and trans dienophiles generate trans substitution.
- The diene in a Diels-Alder reaction must be able to adopt an s-cis conformation.
- Diels-Alder reactions with cyclic dienes favor endo substituents.
14.6 Diene Polymers - Natural and Synthetic Rubbers
- Dienes can form polymers including natural examples such as rubber which is formed from isoprene monomers.
- When UV and visible light is absorbed, electrons are excited from a bonding or non-bonding orbital to a nearby anti-bonding orbital.
- Colored organic molecules all include extensive conjugated pi electron systems.
- UV absorbance is higher energy than visible light, allowing for excitation of electrons without the low energy pi-bonding to pi anti-bonding transitions available in highly conjugated molecules.
- Chromophores are molecules or structural features that absorb light in the UV-Visible range.
14.8 Interpreting Ultraviolet Spectra: The Effect of Conjugation
- Conjugated pi systems lowers the energy gap for π -π* transitions causing the molecule to absorb light of a longer wavelength.
- Many molecules absorb in the UV spectrum. As the energy gap becomes smaller, these absorbances move into the visible spectrum, starting with violet light which makes the resulting molecules appear yellow.
14.9 Conjugation, Color, and the Chemistry of Vision
- Absorption of light causes chemical changes in the rods and cones in eyes leading to visual sensations.
Skills to Master
- Skill 14.1 Differentiate between conjugated and isolated dienes.
- Skill 14.2 Explain stability of conjugated dienes using Molecular Orbital Theory.
- Skill 14.3 Draw mechanisms for 1,2 and 1,4 addition to conjugated dienes.
- Skill 14.4 Predict kinetic and thermodynamic products of addition reactions to conjugated dienes.
- Skill 14.5 Explain kinetic and thermodynamic control of reactions.
- Skill 14.6 Identify dienes and dienophiles for Diels-Alder Cycloaddition reactions.
- Skill 14.7 Determine products of Diels-Alder Cycloaddition, including stereochemistry and endo/exo.
- Skill 14.8 Draw mechanisms for Diels-Alder Cycloaddition.
- Skill 14.9 Apply reactions of conjugated dienes to polymerization and natural rubber formation.
- Skill 14.10 Explain the electronic transitions that occur with absorption of UV-Visible light.
- Skill 14.11 Explain the effects of conjugation on wavelength of light absorbed in π -π* transitions.
Summary of Reactions
Electrophilic Addition
Diels−Alder Cycloaddition